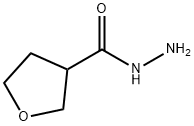
Tetrahydro-3-furancarboxylic acid hydrazide synthesis
- Product Name:Tetrahydro-3-furancarboxylic acid hydrazide
- CAS Number:59293-32-2
- Molecular formula:C5H10N2O2
- Molecular Weight:130.15
89364-31-8

59293-32-2
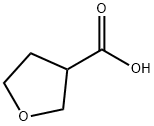
Example 6.1 (1014): isobutyl chloroformate (2.86 mL, 23.00 mmol) was added slowly and dropwise to a solution of tetrahydrofuran (THF, 42 mL) containing tetrahydro-3-furancarboxylic acid (2.0 mL, 20.9 mmol) and triethylamine (TEA, 5.8 mL, 42 mmol) at 0 °C. The reaction mixture was stirred continuously at 0 °C for 1 hour. Subsequently, hydrazine (0.67 mL, 23.00 mmol) was added to the reaction system and the mixture was continued to be stirred at room temperature for 1 hour. Upon completion of the reaction, the mixture was concentrated to remove the solvent. Chloroform (200 mL) and water (20 mL) were added to the residue to promote dissolution. The organic layer was separated and the aqueous phase was processed by lyophilization. The solid fraction was ground three times with ethanol (EtOH, 50 mL x 3). The ethanol solutions were combined, concentrated and dried to give the final tetrahydro-3-furancarbohydrazide (2.27 g). The product was detected by liquid chromatography-mass spectrometry (LCMS) electrospray ionization (ESI) in positive ion mode, m/z = 131.1 (M + H)+).

89364-31-8
204 suppliers
$8.00/1g

59293-32-2
45 suppliers
inquiry
Yield: 2.27 g
Reaction Conditions:
Stage #1:3-tetrahydrofuroic acid with triethylamine;isobutyl chloroformate in tetrahydrofuran at 0; for 1 h;
Stage #2: with hydrazine in tetrahydrofuran at 20; for 1 h;
Steps:
6.0.6.1 (S)-Tetrahydrofuran-3-carbohydrazide and (R)-tetrahydrofuran-3-carbohydrazide, Example 6.1
(S)-Tetrahydrofuran-3-carbohydrazide and (R)-tetrahydrofuran-3-carbohydrazide, Example 6.1 (1014) To a mixture of tetrahydro-3-furanoic acid (2.0 mL, 20.9 mmol) and TEA (5.8 mL, 42 mmol) in THF (42 mL) was added dropwise isobutyl chloroformate (2.86 mL, 23.00 mmol). The resulting mixture was stirred at 0° C. for 1 h before hydrazine (0.67 mL, 23.00 mmol) was added. The resulting mixture was allowed to stir at RT for 1 h. The mixture was then concentrated, and chloroform (200 mL) and water (20 mL) were added to dissolve the residue. The organic layer was separated, and the aqueous phase was lyophilized. EtOH (50 mL×3) was used to triturate the solid 3 times. The combined EtOH solution was concentrated and dried to give tetrahydrofuran-3-carbohydrazide (2.27 g). LCMS ESI (pos) m/z=131.1 (M+H)+.
References:
AMGEN INC.;CHEN, Xiaoqi;CHEN, Yinhong;CHENG, Alan C.;CONNORS, Richard V.;DEBENEDETTO, Mikkel V.;DRANSFIELD, Paul John;FU, Zice;HARVEY, James S.;HEATH, Julie Anne;HEDLEY, Simon J.;HOUZE, Jonathan;JUDD, Ted C.;KHAKOO, Aarif Yusuf;KOPECKY, David John;LAI, Su-Jen;MA, Zhihua;NISHIMURA, Nobuko;OLSON, Steven H.;PATTAROPONG, Vatee;SWAMINATH, Gayathri;WANG, Xiaodong;YEH, Wen-Chen US2017/320860, 2017, A1 Location in patent:Paragraph 1013; 1014
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

59293-32-2
45 suppliers
inquiry