
tert-Butylhydroquinone synthesis
- Product Name:tert-Butylhydroquinone
- CAS Number:1948-33-0
- Molecular formula:C10H14O2
- Molecular Weight:166.22

123-31-9
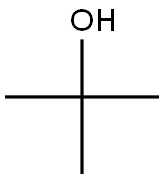
75-65-0

1948-33-0
1) add 3 wt. parts of phosphoric acid and 0.1 wt. parts of water into a stirring cylinder, followed by adding 6 wt. parts of hydroquinone, and start the stirrer for mixing; 2) transfer the mixed solution into a reactor, and add tertiary butanol slowly after heating up to 59°C; 3) continue to heat up to 81°C, and maintain the reaction at this temperature for 2 hrs; 4) after completion of the reaction, centrifuge the product, and then wash and dehydrate it to obtain TBHQ crude oil; 5) mix 1 wt. part of acid with 95 wt. part of water, heat to 81°C, then add 6.5 wt. part of TBHQ crude oil, and heat to 95°C with stirring; 6) carry out a first centrifugation of the solution of step 5) to isolate DTBHQ insoluble in water, and then subsequently cool down the solution to below 45°C, carry out a second centrifugation, and the TBHQ crystals obtained are washed, dehydration and then dried; during the drying process, the wet TBHQ crystals were uniformly spread on a vibrating screen, sieved and then flatly spread on a drying disk with the thickness controlled at 5 cm, and dried for 8 hours, and ultimately obtained a TBHQ boutique product with a TBHQ content of 99.9% and a DTBHQ content of 0.1%.

123-31-9
855 suppliers
$13.00/25g

75-65-0
785 suppliers
$10.00/10ml

1948-33-0
695 suppliers
$5.00/25g
Yield:1948-33-0 99.9%
Reaction Conditions:
with phosphoric acid in water at 59 - 81; for 2 h;Temperature;
Steps:
7 The preparation process of the TBHQ boutique of the present embodiment includes the following steps
1) 3 parts by weight of phosphoric acid, 0.1 part by weight of water was poured into a stirring cylinder, and 6 parts by weight of hydroquinone was added to the stirring cylinder to carry out stirring;2) After stirring, the mixed solution was placed in a reaction kettle and heated to 59 ° C, and then the reaction vessel was charged with t-butanolCopies;3) heating to 81 ° C, the insulation for 2 hours to react;4) the reaction product by centrifugal separation, washing, dehydration after TBHQ crude;5) 1 part by weight of acid and 95 parts by weight of water were mixed and then heated. After heating to 81 ° C, 6.5 parts by weight of TBHQ was added and heated to 95 ° C with stirring.6) The solution of step 5) was centrifuged for the first time, the DTBHQ insoluble in water in the crude TBHQ was separated, and then the solution was cooled. When the solution was cooled to 45C or lower, the second centrifugation was carried out, and the obtained TBHQ crystal washing, dehydration, drying, that was TBHQ boutique.Wherein the drying in step 6) is carried out by pouring the wet TBHQ boutique into a vibrating screen sieve, laying the sieved TBHQ boutique on a drying tray, having a thickness of 5 cm and drying for 8 hours.The TBHQ was obtained, where the weight percentage of TBHQ was 99.9% and the weight percentage of DTBHQ was 0.1%.
References:
CN103864580,2016,B Location in patent:Paragraph 0095-0104
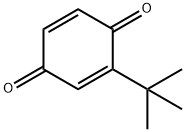
3602-55-9
175 suppliers
$70.00/500mg

1948-33-0
695 suppliers
$5.00/25g

123-31-9
855 suppliers
$13.00/25g

75-65-0
785 suppliers
$10.00/10ml

5875-45-6
20 suppliers
inquiry

1948-33-0
695 suppliers
$5.00/25g
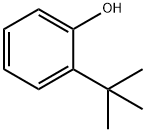
88-18-6
188 suppliers
$16.00/25mL

3602-55-9
175 suppliers
$70.00/500mg

1948-33-0
695 suppliers
$5.00/25g

540-88-5
388 suppliers
$22.00/25mL

123-31-9
855 suppliers
$13.00/25g

1948-33-0
695 suppliers
$5.00/25g