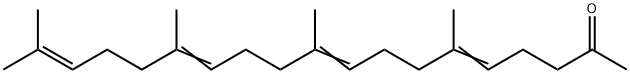
Teprenone synthesis
- Product Name:Teprenone
- CAS Number:6809-52-5
- Molecular formula:C23H38O
- Molecular Weight:330.55
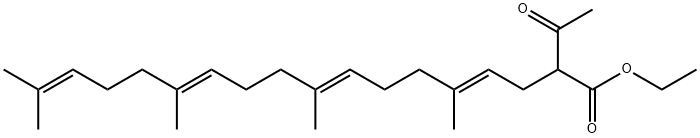
71668-81-0

6809-52-5
The 3-racemic-keto ester 11 (0.07 g, 0.174 mmol) was used as a raw material, which was mixed with MeOH (0.3 mL) and 5N KOH aqueous solution (0.15 mL), and heated to react at 80-85 °C for 2 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, the reaction mixture was cooled and acidified with 2N HCl, followed by extraction with ether, ethyl acetate or hexane (3 x 400 mL). The organic layers were combined and washed sequentially with water, aqueous NaHCO3 and brine, and then dried with anhydrous MgSO4. After removing the solvent under reduced pressure, the oily crude product obtained was purified by silica gel column chromatography with a gradient elution of hexane to 1-2% EtOAc in hexane solution as eluent to end up with the colorless liquid 5-trans-GGA 12. Yield: 0.028 g (50%). TLC Rf value: 0.45 (5% EtOAc/hexane); LCMS data: MS (m/z): 333 (MH+); 353 (M+Na).

71668-81-0
5 suppliers
inquiry

6809-52-5
206 suppliers
$25.00/5mg
Yield:6809-52-5 50%
Reaction Conditions:
with potassium hydroxide in methanol;water at 80 - 85; for 2 h;
Steps:
1 5E,9E,13E-Geranylgeranyl acetone 12 (5-trans-GGA)
A mixture of 3-rac-ketoester 11, (0.07 g, 0.174 mmol) MeOH (0.3 mL), and 5N aqueous KOH (0.15 mL) was heated at 80-85° C. for 2 h, reaction was followed by TLC.
After cooling the reaction mixture, it was acidified with 2N HCl and extracted with diethyl ether, ethyl acetate or hexanes (3*400 mL).
The combined organic layers were successively washed with water, aqueous NaHCO3, brine and dried over anhydrous MgSO4.
After removal of solvent, the oily crude product was purified by silica gel column chromatography using n-hexanes to 1-2% EtOAc in n-Hexanes to afford a colorless liquid of 5-trans-GGA 12. Yield: 0.028 g (50%). TLC Rf: 0.45 (5% EtOAc/n-hexanes); LCMS: MS (m/z): 333 (MH+); 353 (M+Na).
References:
Coyote Pharmaceuticals, Inc.;Serizawa, Hiroaki;Argade, Ankush B.;Datwani, Akash;Spencer, Natalie US2013/85283, 2013, A1 Location in patent:Paragraph 0180
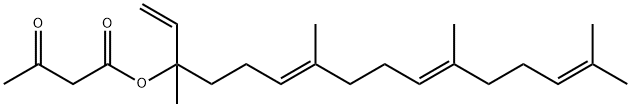
1206897-24-6
0 suppliers
inquiry

6809-52-5
206 suppliers
$25.00/5mg
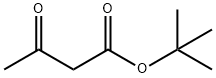
1694-31-1
385 suppliers
$6.00/25g
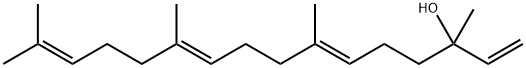
1113-21-9
191 suppliers
$6.00/1g

6809-52-5
206 suppliers
$25.00/5mg

1113-21-9
191 suppliers
$6.00/1g
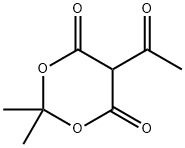
72324-39-1
57 suppliers
$130.00/500mg

6809-52-5
206 suppliers
$25.00/5mg
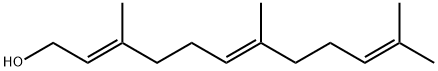
106-28-5
247 suppliers
$10.00/1g

6809-52-5
206 suppliers
$25.00/5mg