
TAK-632 synthesis
- Product Name:TAK-632
- CAS Number:1228591-30-7
- Molecular formula:C27H18F4N4O3S
- Molecular Weight:554.52
![N-(5-(2-aMino-7-cyanobenzo[d]thiazol-6-yloxy)-2-fluorophenyl)-2-(3-(trifluoroMethyl)phenyl)acetaMide](/CAS/20150408/GIF/1228591-34-1.gif)
1228591-34-1
0 suppliers
inquiry
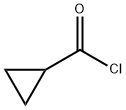
4023-34-1
368 suppliers
$12.00/5g

1228591-30-7
119 suppliers
$25.00/1mg
Yield: 95%
Reaction Conditions:
with pyridine in tetrahydrofuran at 20; for 2 h;
Steps:
53.v
To a solution of N- {5- [ (2-amino-7-cyano-l, 3-benzothiazol- 6-yl ) oxy] -2-fluorophenyl } -2- [ 3-(trifluoromethyl) phenyl ] acetamide (980 mg, 2.01 mmol) in N, N- dimethylacetamide (8 inL) were added pyridine (242 μL, 3.02 mmol) and cyclopropanecarbonyl chloride (255 μL, 2.81 mmol), and the mixture was stirred at room temperature for 2 hr. Cyclopropanecarbonyl chloride (255 μL, 2.81 mmol) was added to the reaction mixture, and the mixture was further stirred at room temperature for 2 hr. Water (20 mL) was added to the reaction mixture, and the mixture was extracted with ethyl acetate (20 mL) . The organic layer was washed successively with saturated aqueous sodium hydrogen carbonate solution (20 mL) and saturated brine (20 mL) , and dried over anhydrous magnesium sulfate. Insoluble material was filtered off, the filtrate was purified by basic silica gel column chromatography (eluate: ethyl acetate) , and the obtained solution was concentrated under reduced pressure. A pale-brown oil residue was crystallized from ethanol/water (1/1) to give the title compound (1.06 g, 95%) as a white powder. 1H-NMR (DMSO-d6, 300 MHz) δ 0.89 - 1.05 (4H, m) , 1.97 - 2.13 (IH, m) , 3.88 (2H, s) , 6.97 (IH, dt, J = 8.7, 3.6 Hz), 7.08 (IH, d, J = 9.0 Hz), 7.37 (IH, dd, J = 10.6, 9.1 Hz), 7.49 - 7.64 (3H, m) , 7.68 (IH, s) , 7.83 (IH, dd, J = 6.4, 3.0 Hz), 7.99 (IH, d, J = 9.0 Hz), 10.21 (IH, s) , 12.97 (IH, s) .
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED;OKANIWA, Masanori;TAKAGI, Terufumi WO2010/64722, 2010, A1 Location in patent:Page/Page column 192
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
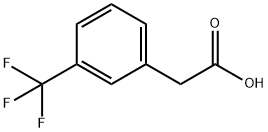
351-35-9
244 suppliers
$9.00/5g

1228591-30-7
119 suppliers
$25.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1228591-30-7
119 suppliers
$25.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1228591-30-7
119 suppliers
$25.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1228591-30-7
119 suppliers
$25.00/1mg