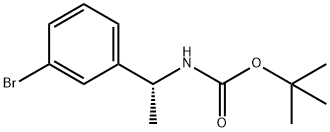
(R)-tert-butyl 1-(3-broMophenyl)ethylcarbaMate synthesis
- Product Name:(R)-tert-butyl 1-(3-broMophenyl)ethylcarbaMate
- CAS Number:1187932-25-7
- Molecular formula:C13H18BrNO2
- Molecular Weight:300.19
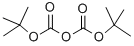
24424-99-5
864 suppliers
$13.50/25G
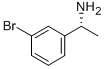
176707-77-0
92 suppliers
$40.00/1g

1187932-25-7
40 suppliers
$90.00/50mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20;
Steps:
16
Example 16, Compound 16; (E)-(2R,5S,11 S,14S,17R,18R)-11 -(3-Hydroxy-benzyl)-14-isopropyl-18-methoxy- 2,17-dimethyl-3,9,12,15,28-pentaaza-tricyclo[21.3.1.1 *5,9*]octacosa- 1 (27),21 ,23,25-tetraene-4,10,13,16-tetraone.; Com ound 16a: [(R)-1 -(3-Bromo-phenyl)-ethyl]-carbamic acid iert-butyl esterA solution of (R)-bromo-a-methylbenzylamine (1 .023 g, 5.1 12 mmol) indichloromethane (20 mL) was subsequently treated with triethylamine (720 μΙ_, 5.1 12 mmol) and di-te/t-butyl dicarbonate (1 .784 g, 8.179 mmol). After overnight stirring at room temperature, the volatiles were removed in vacuo and the residue was purified by silica gel chromatography using a 50 g Isolute cartridge eluted with a continuous gradient of /so-hexanes/Et20 1 :0 to 4:1 to afford the title compound (1 .552 g, 100%) as a white solid. H NMR (300 MHz, CDCI3) 1 .43 (br s, 12H), 4.77 (br s, 2H), 7.16-7.26 (m, 2H), 7.39 (dt, J = 2.0, 7.1 Hz, 1 H), 7.46 (s, 1 H).
References:
GILEAD SCIENCES, INC.;SELCIA LIMITED;APPLEBY, Todd;FLIRI, Hans, G.;KEATS, Andrew, J.;LAZARIDES, Linos;MACKMAN, Richard, L.;PETTIT, Simon, N.;POULLENNEC, Karine, G.;SANVOISIN, Jonathan;STEADMAN, Victoria, A.;WATT, Gregory, M. WO2012/78915, 2012, A1 Location in patent:Page/Page column 90