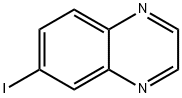
Quinoxaline, 6-iodo- (9CI) synthesis
- Product Name:Quinoxaline, 6-iodo- (9CI)
- CAS Number:50998-18-0
- Molecular formula:C8H5IN2
- Molecular Weight:256.04

131543-46-9

21304-38-1

50998-18-0
The reaction was carried out with 4-iodo-1,2-benzenediamine (0.46 g, 1.96 mmol) and glyoxal (40% aqueous solution, 2.25 mL) in a solvent mixture of acetic acid (1 mL) and ethanol (20 mL), heated at 100 °C for several hours. After completion of the reaction, the mixture was cooled to room temperature. Subsequently, water was added for dilution and the crude product was extracted with ethyl acetate. The target compound 6-iodoquinoxaline (0.323 g, 64% yield) was purified by silica gel column chromatography using hexane and ethyl acetate (1:1, v/v) as eluent. The product was characterized by 1H NMR (400 MHz, CDCl3) with chemical shifts (δ) of 8.77 (dd, 2H, J = 2.0, 8.8 Hz), 8.46 (d, 1H, J = 2.0 Hz), 7.96 (dd, 1H, J = 2.0, 8.8 Hz), 7.75 (d, 1H, J = 8.8 Hz).

131543-46-9
1 suppliers
inquiry

21304-38-1
107 suppliers
$36.00/250mg

50998-18-0
56 suppliers
$69.00/100mg
Yield:50998-18-0 64%
Reaction Conditions:
with acetic acid in ethanol;water at 100;
Steps:
71.a
A solution of 4-iodo-benzene-l,2-diamJne (0.46g, 1.96mmol), ethanedial [40% in water] (2.25mL), acetic acid (ImL) and ethanol (2OmL) were heated to 1000C for several hours and then cooled to room temperature. Water was added and the crude product was extracted with ethyl acetate. The product was purified via silica gel column chromatography with hexane: ethyl acetate (1:1) to give 0.323g (64%) of 6- iodo-quinoxaline. 1H NMR (400 MHz, CDCl3) S 8.77 (dd, 2H, J=2.0, 8.8Hz), 8.46 (d, IH, 2.0Hz), 7.96 (dd, IH, J=2.0, 8.8Hz), 7.75 (d, IH, J=8.8Hz).
References:
WO2007/75567,2007,A1 Location in patent:Page/Page column 131

21304-38-1
107 suppliers
$36.00/250mg
517-21-5
95 suppliers
$17.00/25g

50998-18-0
56 suppliers
$69.00/100mg