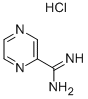
PYRAZINE-2-CARBOXAMIDINE HYDROCHLORIDE synthesis
- Product Name:PYRAZINE-2-CARBOXAMIDINE HYDROCHLORIDE
- CAS Number:138588-41-7
- Molecular formula:C5H7ClN4
- Molecular Weight:158.59
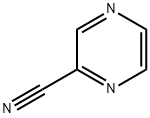
19847-12-2

138588-41-7
The general procedure for the synthesis of 2-pyrazinamidine hydrochloride from 2-cyanopyrazine was as follows: 2-cyanopyrazine (1.00 kg, 9.53 mol) was slowly added to a solution of sodium methanol (NaOCH3, 51.4 g, 0.952 mol) in methanol (3800 mL) at room temperature. The reaction mixture was heated to 30 °C and stirred for 6 hours. Upon completion of the reaction, the mixture was cooled to 25 °C, followed by the addition of ammonium chloride (NH4Cl, 572 g, 10.5 mol). Stirring of the reaction mixture was continued for 22 hours. At the end of the reaction, methyl tert-butyl ether (4000 mL) was added and stirred for 15 minutes to promote solid formation. The solid product was collected by filtration and washed with methyl tert-butyl ether (2 x 1000 mL). Finally, the product was dried at 40 °C, 0-10 mmHg for 17 h to give the white solid product 2-pyrazinamidine hydrochloride (1435 g) in 95% yield and 95% purity by HPLC.1H NMR (DMSO-D6) data were as follows: δ 9.7 (broad single peak, 3H), 9.49 (double peak, 1H, J=1.5Hz), 9.04 (multiple peaks, 1H), 8.93 (multiple peaks, 1H), 9.04 (multiple peaks, 1H), 8.93 (multiple peaks 1H), 8.93 (triple peak, 1H, J=1.5Hz).

19847-12-2
357 suppliers
$6.00/5g

138588-41-7
97 suppliers
$13.00/1g
Yield: 95%
Reaction Conditions:
Stage #1:2-pyrazine carbonitrile with sodium methylate in methanol at 20 - 30; for 6 h;
Stage #2: with ammonium chloride at 25; for 22 h;
Steps:
1; 1.a Pyrazine-2-carboxamidine hydrochloride
To a solution of sodium methoxide (NaOCH3) (51.4 g, 0.952 mol) in methanol (3800 ml) there is added cyanopyrazine (1.00 KG, 9.53 mol) slowly at room temperature. The mixture is heated to 30° C. and stirred for 6 h. The mixture is cooled to 25° C. followed by the addition of ammonium chloride (NH4Cl) (572 g, 10.5 mol). The reaction mixture is stirred for 22 h and methyl t-butyl ether (4000 mL) is added and the mixture is stirred for 15 min forming a solid. The solid is filtered and washed with methyl t-butyl ether (2×1000 mL) then dried at 40° C./0-10 mmHg for 17 h to give white solid product (1435 g) in 95% yield with 95% HPLC purity. 1H NMR (DMSO-D6): δ 9.7 (bs, 3H), 9.49 (d, 1H, J=1.5 Hz), 9.04 (m, 1H), 8.93 (t, 1H, J=1.5 Hz)
References:
Wyeth US2006/281760, 2006, A1 Location in patent:Page/Page column 5; 8

67-56-1
781 suppliers
$7.29/5ml-f

19847-12-2
357 suppliers
$6.00/5g

138588-41-7
97 suppliers
$13.00/1g