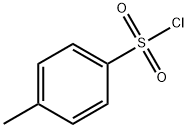
PROPYL P-TOLUENESULFONATE synthesis
- Product Name:PROPYL P-TOLUENESULFONATE
- CAS Number:599-91-7
- Molecular formula:C10H14O3S
- Molecular Weight:214.28

71-23-8
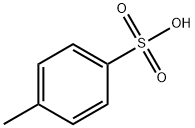
104-15-4

599-91-7
1. Install a thermometer and set up magnetic stirring in a 100mL three-neck flask. Add 20 mL of dichloromethane and cool the reaction flask in an ice bath to an internal temperature of 0-5°C. 2. p-Toluenesulfonic acid monohydrate (380.44 mg, 2.0 mmol), bis(trichloromethyl)carbonate (236 mg, 0.80 mmol, 0.40 equiv), and potassium phosphate trihydrate (K3PO4-3H2O, 1.38 g, 6 mmol, 3 equiv) were added to the reaction flask sequentially, and the flask was stirred for 5 minutes. 3. Two drops of triethylamine (~20 mg, 0.10 equiv) were added dropwise to the reaction vial and after bubbles were observed, the ice bath was removed and the reaction mixture was allowed to warm up to room temperature. Stirring was continued for 20 minutes. 4. n-Propanol (120.2 mg, 2.0 mmol), triethylamine (202.4 mg, 2.0 mmol) and trimethylamine hydrochloride (13.1 mg, 0.2 mmol) were added sequentially and the mixture was stirred for 30 min at room temperature. The reaction process was monitored by TLC. 5. Upon completion of the reaction, the reaction mixture was filtered and the filter cake was washed with 5 mL of dichloromethane to remove unreacted salt. 6. The filtrate was concentrated to dryness under reduced pressure to give 386 mg of propyl p-toluenesulfonate (yield: 90%).
Yield:599-91-7 95%
Reaction Conditions:
with triethylamine in dichloromethane at 5 - 22; for 12.5 h;Industry scale;
Steps:
16
[00263] EXAMPLE 16 - Industrial scale preparation of propyl tosylate:; A 100 L glass, jacketed reactor was charged with 1-propanol (2.098 kg; 34.9 mol), triethylamine (4.585 kg; 45.3 mol; 1.3 equivalents) and DCM (20.1 L). The mixture was cooled to a temperature of about 5oC to 15oC and cautiously charged with a solution of /Moluenesulfonyl chloride (6 kg; 31.47 mol; 0.9 equivalents) in DCM (10.5 L) over 30 minutes. Once the addition was complete, the mixture was warmed to a temperature of about 18oC to 22oC and stirred for 12 hours. The reaction mixture was assayed by 1H NMR (in CDCI3) and deemed complete. HCl (6 N; 2.98 L) was cautiously charged while maintaining the temperature below 25oC. The aqueous phase was removed, and the organic phase was washed 2 X with water (21 L each wash), dried with MgSC>4, and filtered over Celite. The filtered solids were then washed with DCM (4 L) and concentrated to a residue. The residue was dissolved in heptane and concentrated again to afford a final propyl tosylate product (6.385 kg, 95% yield).
References:
WO2010/22140,2010,A1 Location in patent:Page/Page column 101

71-23-8
788 suppliers
$12.00/100g

104-15-4
755 suppliers
$133.00/500g

599-91-7
129 suppliers
$6.00/5g

104-15-4
755 suppliers
$133.00/500g

143885-02-3
0 suppliers
inquiry
22731-83-5
22 suppliers
inquiry

599-91-7
129 suppliers
$6.00/5g