
Pivaloylacetonitrile synthesis
- Product Name:Pivaloylacetonitrile
- CAS Number:59997-51-2
- Molecular formula:C7H11NO
- Molecular Weight:125.17

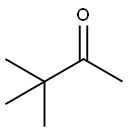
598-98-1
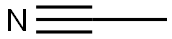
75-05-8

59997-51-2
1. NaH (50% in paraffin oil, 1.2 eq., 4.6 g) was added to 1,4-dioxane (120 ml) and stirred for several minutes to disperse the mixture. 2. Acetonitrile (1.2 eq., 4.2 g) was slowly added dropwise to the above mixture over a period of 15 minutes and stirring was continued for 30 minutes after completion of the addition. 3. Methyl pivalate (1 eq., 10 g) was added slowly and dropwise to the reaction system over 15 minutes, after which the reaction mixture was heated to reflux for 3 hours. 4. After completion of the reaction, the reaction mixture was poured into ice water (200 g) and the pH was adjusted to 4.5 with acid. 5. The aqueous phase was extracted several times with dichloromethane (12 x 250 ml) and the organic phase was combined. 6. The combined organic phases were dried with anhydrous sodium sulfate, filtered and concentrated. 7. The concentrate was recrystallized with hexane (100 ml) to give the brown solid product 4,4-dimethyl-3-oxopentanenitrile (5 g, 51% yield).
75-97-8
245 suppliers
$10.00/1g

59997-51-2
269 suppliers
$6.00/25g
Yield:59997-51-2 85%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;sodium bicarbonate;chlorine in methanol;water;benzene
Steps:
2 EXAMPLE 2
EXAMPLE 2 To a solution of pinacolone (100.16 g, 1 mol) in methanol (300 ml) is introduced gaseous chlorine (70.91 g, 1 mol) at 15-20° C. over a period of 1 hour. The reaction mixture is neutralized with a 48% solution of sodium hydroxide in methanol (285 ml) and then aqueous solution of sodium hydrogen carbonate (4.20 g) in that order. After dilution with methanol (40 ml), the resulting mixture is mixed with an aqueous solution (137 ml) of sodium cyanide (58.81 g, 1.2 mol) and refluxed for 1 hour. The methanol is evaporated under atmospheric pressure over a period of 1.5 hours. The residue is mixed with a 4% aqueous sodium hydroxide (650 g) solution and shaken with benzene (300 ml) to remove neutral material (10.78 g). Hydrochloric acid (35%) (92.60 g) is poured into said alkaline aqueous layer below 15° C., and the mixture is shaken with benzene (750 ml). The benzene layer is washed with water, then mixed with an aqueous solution (44 ml) of sodium hydrogen carbonate (0.66 g), and the benzene layer is evaporated under atmospheric pressure. A solution of the residue is adjusted to pH 7.0 by stirring with benzene (142 ml), water (30 ml) then 35% hydrochloric acid (0.37 g). The benzene layer is dried and concentrated to give cyanopinacolone (106.70 g) as crystals melting at 64° to 67° C. Yield is 85 %.
References:
Shionogi & Co., Ltd. US4186144, 1980, A

598-98-1
161 suppliers
$24.00/25mL
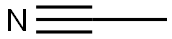
75-05-8
1036 suppliers
$10.00/10g

59997-51-2
269 suppliers
$6.00/25g
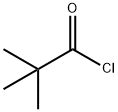
3282-30-2
377 suppliers
$18.00/100ml
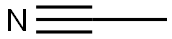
75-05-8
1036 suppliers
$10.00/10g

59997-51-2
269 suppliers
$6.00/25g
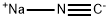
773837-37-9
0 suppliers
inquiry

5469-26-1
181 suppliers
$12.00/1g

59997-51-2
269 suppliers
$6.00/25g

598-98-1
161 suppliers
$24.00/25mL

59997-51-2
269 suppliers
$6.00/25g