
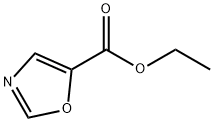
OXAZOLE-5-CARBOXYLIC ACID synthesis
- Product Name:OXAZOLE-5-CARBOXYLIC ACID
- CAS Number:118994-90-4
- Molecular formula:C4H3NO3
- Molecular Weight:113.07
118994-89-1

118994-90-4
General procedure for the synthesis of oxazole-5-carboxylic acid from ethyl oxazole-5-carboxylate: an aqueous solution of lithium hydroxide monohydrate (124.5 kg, prepared by dissolving 49.44 kg of lithium hydroxide monohydrate in 319 kg of water, 398 mol) was slowly added to a solution of ethyl oxazole-5-carboxylate (54 kg, 382.7 mol) in water (54 kg), the reaction The temperature was kept below 25°C during the reaction. the reaction mixture was stirred for 6.5 h and then concentrated. Subsequently, an aqueous HCl solution (64.8 kg) was slowly added while keeping the temperature below 25°C. The mixture was cooled to 5°C and maintained for 1 h to promote crystallization. The crystalline product was collected by filtration, washed sequentially with cold water (88 kg) and isopropanol (171 kg) and finally dried under vacuum at 50 °C to give oxazole-5-carboxylic acid (37.88 kg, 87% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 13.68 (br.s, 1H), 8.59 (s, 1H), 7.88 (s, 1H).

118994-89-1
192 suppliers
$20.00/1g

118994-90-4
141 suppliers
$9.00/250mg
Yield:118994-90-4 87.5%
Reaction Conditions:
with lithium hydroxide monohydrate;water at 25; for 6.5 h;Large scale;
Steps:
Intermediate 1Oxazole-5-carboxvlic acid
An aqueous solution of lithium hydroxide monohydrate (124.5 kg of a solution preparedfrom 49.44 kg lithium hydroxide monohydrate dissolved in 319 kg water, 398 mol) was added to asolution of ethyl 5-oxazolecarboxylate (54 kg, 382.7 mol) in water (54 kg) maintaining the temperature below 25 °C. The reaction was stirred for 6.5 h and then conc. aqueous HCI (64.8 kg) was added maintaining the temperature below 25 °C, the crystallisation cooled to 5 °C and held forh. The product was filtered off, washed with cold water (88 kg), then isopropanol (171 kg) anddried under vacuum at 50 °C to give the title compound (37.88 kg, 87.S%).1H NMR (400 MHz, DMSO-d6) ppm 13.68 (br. 5., 1 H), 8.59 (5, 1 H), and 7.88 (5, 1 H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;BREAM, Robert Nicholas;HAYLER, John David;IRONMONGER, Alan Geoffrey;SZETO, Peter;WEBB, Michael Robert;WHEELHOUSE, Katherine Marie Penelope;WILLACY, Robert David WO2016/193255, 2016, A1 Location in patent:Page/Page column 21