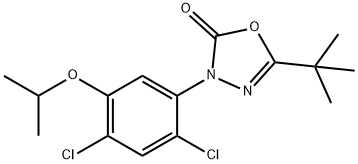
Oxadiazon synthesis
- Product Name:Oxadiazon
- CAS Number:19666-30-9
- Molecular formula:C15H18Cl2N2O3
- Molecular Weight:345.22
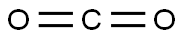
124-38-9
![N-[2,4-Dichloro-5-(1-methylethoxy)phenyl]-2,2-dimethylpropanehydrazonoyl chloride](/CAS/20210305/GIF/51166-88-2.gif)
51166-88-2

19666-30-9
To a 20 mL autoclave was added a stirrer, 0.2 mmol of pivaloyl chloride-2,4-dichloro-5-isopropoxyphenylhydrazone, 0.5 mmol of cesium fluoride, 0.24 mmol of 18-crown-6 and 2 mL of toluene. Carbon dioxide was passed until the pressure reached 2.0 MPa, and the reaction was stirred at 25°C for 24 hours. After the reaction was completed, the heating and stirring were stopped, and the unreacted carbon dioxide was slowly released after the system was cooled to room temperature. The reaction solution was transferred to a partition funnel, 15 mL of water was added and extracted three times with 15 mL of ethyl acetate. The organic phases were combined and washed sequentially with water and saturated saline. The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent to obtain the crude product. The crude product was purified by column chromatography with the eluent of petroleum ether and ethyl acetate (30:1, v/v) to afford the target product 5-(tert-butyl)-3-(2,4-dichloro-5-isopropoxyphenyl)-1,3,4-oxadiazol-2(3H)-one in 88% yield.
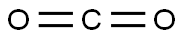
124-38-9
132 suppliers
$175.00/23402
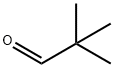
630-19-3
329 suppliers
$13.00/1g
40178-22-1
39 suppliers
inquiry

19666-30-9
213 suppliers
$57.89/100mg
Yield:19666-30-9 72%
Reaction Conditions:
with tert.-butylhydroperoxide;potassium tert-butylate;potassium iodide in water;N,N-dimethyl-formamide at 80; under 15001.5 Torr; for 8 h;Autoclave;
References:
Yang, Na;Yuan, Gaoqing [Organic and Biomolecular Chemistry,2019,vol. 17,# 27,p. 6639 - 6644]