
O-BENZYL-L-SERINE synthesis
- Product Name:O-BENZYL-L-SERINE
- CAS Number:4726-96-9
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
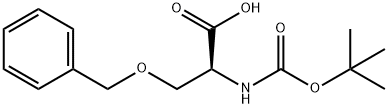
23680-31-1

4726-96-9
The general procedure for the synthesis of (S)-2-amino-3-(benzyloxy)propionic acid from N-BOC-O-benzyl-L-serine was as follows: the compound obtained in the previous step was dissolved in an appropriate amount of dry dichloromethane (DCM), 1.0 mL of trifluoroacetic acid (TFA) and 0.1 mL of triethylsilane were added, and the reaction was stirred for 2 hr at room temperature, and the reaction was monitored by thin-layer chromatography (TLC) The reaction was monitored until complete. Upon completion of the reaction, the pH of the reaction solution was adjusted to 7?8 with saturated sodium bicarbonate solution and subsequently diluted with the addition of dichloromethane (DCM). The organic phase was washed twice with saturated brine and then concentrated to give a yellow oily liquid. Finally, the product was purified by silica gel column chromatography (eluent ratio of chloroform:methanol=15:1) to give a yellow powdery solid product in 62.3% yield.
![L-Serine, N-[[(4-methoxyphenyl)methoxy]carbonyl]-O-(phenylmethyl)-](/CAS/20210305/GIF/4218-63-7.gif)
4218-63-7
0 suppliers
inquiry

4726-96-9
219 suppliers
$6.00/1g
Yield:4726-96-9 100%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane; for 0.166667 h;
References:
Chen, Shui-Tein;Wang, Kung-Tsung [Synthesis,1989,# 1,p. 36 - 37]

23680-31-1
289 suppliers
$7.00/5g

4726-96-9
219 suppliers
$6.00/1g

20806-43-3
130 suppliers
$6.00/250mg

4726-96-9
219 suppliers
$6.00/1g

20806-43-3
130 suppliers
$6.00/250mg
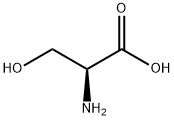
56-45-1
795 suppliers
$5.00/25g

4726-96-9
219 suppliers
$6.00/1g

5445-44-3
148 suppliers
$10.00/1g

4726-96-9
219 suppliers
$6.00/1g
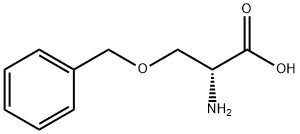
10433-52-0
168 suppliers
$8.00/250mg