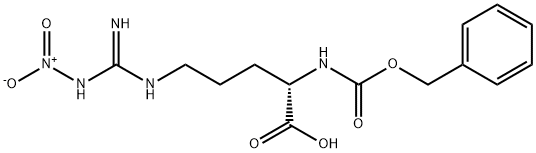
Nalpha-Cbz-L-Arginine synthesis
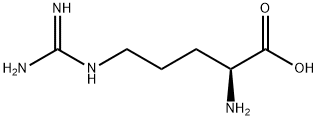
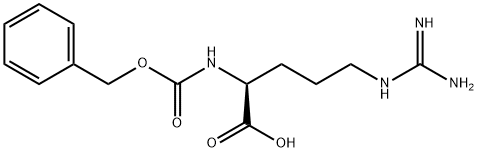
- Product Name:Nalpha-Cbz-L-Arginine
- CAS Number:1234-35-1
- Molecular formula:C14H20N4O4
- Molecular Weight:308.33
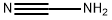
420-04-2
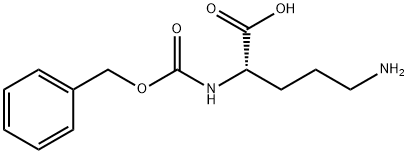
2640-58-6

1234-35-1
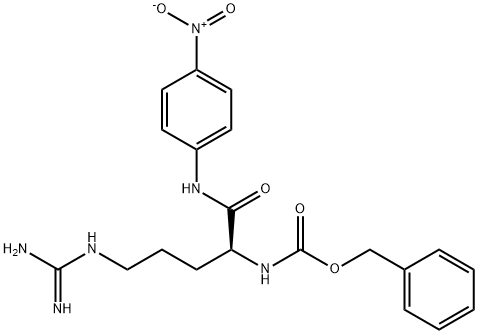
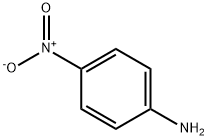
The general procedure for the synthesis of Cbz-arginine from cyanamide and (S)-5-amino-2-(benzyloxycarbonyloxyamino)pentanoic acid was as follows: to a solution of water (2.5 mL) containing p-methoxyaniline (1b, 61.6 mg, 0.50 μmol) and cyanamide (25.2 mg, 0.60 μmol) was added scandium trifluoromethanesulfonate (Sc(OTf)3, at room temperature. 24.6 mg, 50 nmol). The reaction mixture was stirred at 100 °C for 2 days. After completion of the reaction, the reaction mixture was washed with chloroform (3 x 10 mL) extraction. The aqueous layer was separated and concentrated under vacuum. The residue was purified by filtration through a silica gel pad (eluent: chloroform-methanol, 20:1, v/v).
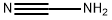
420-04-2
379 suppliers
$15.00/25g

2640-58-6
130 suppliers
$7.00/250mg

1234-35-1
273 suppliers
$5.00/1g
Yield:1234-35-1 68%
Reaction Conditions:
with scandium tris(trifluoromethanesulfonate) in water at 100; for 24 h;
Steps:
General Procedure for the Guanylation of Amines:
General procedure: To a solution of p-methoxyaniline (1b, 61.6 mg, 0.50 μmol)and cyanamide (25.2 mg, 0.60 μmol) in H2O (2.5 mL) wasadded Sc(OTf)3 (24.6 mg, 50 nmol) at r.t. After the solutionwas stirred at 100 °C for 2 d, the resulting mixture waswashed with CHCl3 (3 × 10 mL). The aqueous layer wasconcentrated in vacuo, and the residue was purified by filtering through silica gel pad (CHCl3-MeOH, 20:1).
References:
Tsubokura, Kazuki;Iwata, Takayuki;Taichi, Misako;Kurbangalieva, Almira;Fukase, Koichi;Nakao, Yoichi;Tanaka, Katsunori [Synlett,2014,vol. 25,# 9,p. 1302 - 1306]
2304-98-5
133 suppliers
$10.00/1g

1234-35-1
273 suppliers
$5.00/1g
501-53-1
417 suppliers
$10.00/1g

1234-35-1
273 suppliers
$5.00/1g