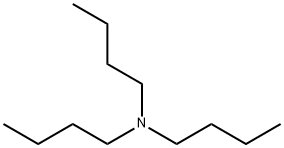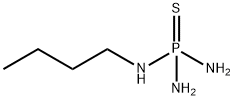
N-(n-Butyl)thiophosphoric triamide synthesis
- Product Name:N-(n-Butyl)thiophosphoric triamide
- CAS Number:94317-64-3
- Molecular formula:C4H14N3PS
- Molecular Weight:167.21
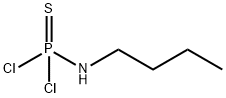
6141-81-7

94317-64-3
General procedure for the synthesis of n-butylthiophosphoric triamine from the compound (CAS: 6141-81-7): first, ammonia was passed through cold hydrazine and externally observed until liquid ammonia was formed. A cooling device was started in a four-necked flask equipped with a stirrer to maintain the reaction temperature at -45°C. Subsequently, 12.75 g of liquid ammonia was added to the flask and the pre-cooled reaction solution was added dropwise stepwise. The dropwise addition process was controlled to be completed within 1.5 hours. Samples were taken during the reaction and when the content of N-butylamino thiophosphoryl dichloride decreased to 2% of the total mass, the reaction temperature was raised to 25°C. The reaction was continued until the substitution reaction was completed, with a total reaction time of 2 h 5 min. At the end of the reaction, the product was filtered, washed and vaporized, and finally recrystallized from n-hexane to obtain N-n-butylaminophosphorothioic acid triamine with a purity of 98.7% and a yield of 95.1%.

109-73-9
468 suppliers
$9.00/1g

94317-64-3
337 suppliers
$6.00/5g
Yield:94317-64-3 89%
Reaction Conditions:
Stage #1:N-butylamine with monothioorthophosphoric acid;dichloromethane;sodium hydroxide at 25; for 0.00555556 h;Large scale;
Stage #2: with ammonia at 15;Large scale;Temperature;
Steps:
25
In this example, 3.46 kg (2.04 mol) of thiophosphoric acid and 2.768 kg of methylene chloride were mixed, and 1.465 kg (2.0 mol) of n-butylamine was added to 2.637 kg of methylene chloride at the same time into a Corning microchannel (2.0 mol) of 10% NaOH solution was injected into the metering plunger pump, and the back pressure valve was adjusted to 0 Pa to control the speed of material entry, and the three materials were uniformly used, and the temperature Control at 25 , the reaction time is 20s.When the material enters the third plate, it is cooled to 15 , and the fourth plate is fed with NH314.96kg (8.8mol) at 15 . The material is separated by gas-liquid separation, and the inorganic layer is separated. After partial distilling off of methylene chloride, the product was cooled and crystallized, and filtered to obtain NBPT. The total yield was 89%, the product mass fraction was over 98%, and the melting point was 57.8 -58.5
References:
Zhejiang Aofutuo Chemical Co., Ltd.;Dai, Feng;Dai, jiaben;Dai, shutian;Dai, yineng CN105254664, 2016, A Location in patent:Paragraph 0062; 0063

6141-81-7
0 suppliers
inquiry

94317-64-3
337 suppliers
$6.00/5g

6141-81-7
0 suppliers
inquiry

94317-64-3
337 suppliers
$6.00/5g
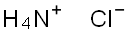
12125-02-9
1304 suppliers
$10.00/250G