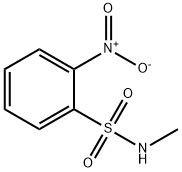
N-Methyl-2-nitrobenzenesulphonamide synthesis
- Product Name:N-Methyl-2-nitrobenzenesulphonamide
- CAS Number:23530-40-7
- Molecular formula:C7H8N2O4S
- Molecular Weight:216.21
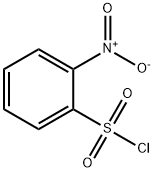
1694-92-4

74-89-5

23530-40-7
Step 2. Synthesis of N-methyl-2-nitrobenzenesulfonamide 2-Nitrobenzene-1-sulfonyl chloride (4 g, 18.05 mmol) was dissolved in dichloromethane (DCM, 60.2 mL) and cooled to 0 °C in an ice water bath. Subsequently, triethylamine (TEA, 7.55 mL, 54.1 mmol) and tetrahydrofuran solution of 2M methylamine (13.54 mL, 27.1 mmol) were added sequentially to this solution. The reaction mixture was stirred at room temperature for 6 hours. After completion of the reaction, the reaction mixture was diluted with dichloromethane and washed with saturated aqueous sodium bicarbonate (NaHCO3) solution (2 x 100 mL) followed by brine (100 mL). The organic layer was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Finally, the concentrate was ground in ether to give N-methyl-2-nitrobenzenesulfonamide (3.12 g, 14.44 mmol, 80% yield). The product was analyzed by liquid chromatography-mass spectrometry (LCMS), showing a m/z of 217.1 (MH+) and a retention time of 0.53 min.
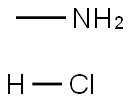
593-51-1
550 suppliers
$21.00/100g

1694-92-4
20 suppliers
$13.31/25G

23530-40-7
68 suppliers
inquiry
Yield:23530-40-7 95%
Reaction Conditions:
with sodium hydroxide in water;ethyl acetate at 0 - 20; for 0.916667 h;
Steps:
N-Methyl-2-nitrobenzenesulfonamide (6)
To a cooled (0 °C) solution of methylamine hydrochloride (15.8 g, 71.3mmol) in H2O (43 mL) was added NaOH (17.2 g, 428 mmol). A solutionof 2-nitrobenzenesulfonyl chloride in EtOAc (140 mL) was addeddropwise via separatory funnel over a period of 30 min. The resultingbiphasic mixture was stirred at 0 °C for 5 additional min and was thenstirred for 20 min at r.t. The layers were then separated and the aqueouslayer was extracted with EtOAc (2 × 100 mL). The combined organiclayers were dried, filtered, and concentrated in vacuo. The residualoff-white solid was crystallized from CH2Cl2/hexanes to affordthe desired product 6 as off-white crystals; yield: 14.6 g (95%).IR (thin film): 3335, 3095, 1540 cm-1.1H NMR (CDCl3, 400 MHz): δ = 8.13 (m, 1 H), 7.86 (m, 1 H), 7.78-7.73(m, 2 H), 5.24 (br s, 1 H), 2.78 (d, J = 4.8 Hz, 3 H).1H NMR was in accordance with literature values.2113C NMR (CDCl3, 100 MHz): δ = 148.2, 133.7, 132.7, 132.4, 131.5 125.4,29.8.LC/MS (ESI): m/z [M - NH(CH3)]+ calcd for C6H5NO4S: 187.1; found:187.1.
References:
Brockway, Anthony J.;Cosner, Casey C.;Volkov, Oleg A.;Phillips, Margaret A.;De Brabander, Jef K. [Synthesis,2016,vol. 48,# 13,art. no. SS-2016-M0105-OP,p. 2065 - 2068]
![2-Cyclopentene-1-carboxamide, 4-(6-chloro-9H-purin-9-yl)-N-[(2-nitrophenyl)sulfonyl]-, (1S,4R)-](/CAS/20210305/GIF/1026978-85-7.gif)
1026978-85-7
0 suppliers
inquiry

23530-40-7
68 suppliers
inquiry