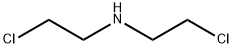
N-CBZ-N,N-BIS(2-CHLOROETHYL)AMINE synthesis
- Product Name:N-CBZ-N,N-BIS(2-CHLOROETHYL)AMINE
- CAS Number:72791-76-5
- Molecular formula:C12H15Cl2NO2
- Molecular Weight:276.16

821-48-7
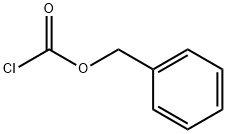
501-53-1

72791-76-5
Bis(2-chloroethyl)amine hydrochloride (5 g, 0.028 mol) was suspended in 30 ml of anhydrous dichloromethane under cooling in an ice water bath and stirring. Benzyl chloroformate (5.1 g, 0.03 mol) was added slowly dropwise with a controlled dropwise time of 10 min. Subsequently, triethylamine (6.46 g, 0.063 mol) was added dropwise over 1 hour. The reaction mixture was continued to be stirred at room temperature for 1 hour. Upon completion of the reaction, the reaction was quenched by the addition of water (10 ml) and stirring was continued for 15 minutes. The organic phase (dichloromethane layer) was separated and washed sequentially with 1M HCl (10 ml) and saturated saline (10 ml), dried anhydrically and concentrated under reduced pressure to give the oily target product benzyl bis(dichloroethyl)aminocarboxylate (7 g, 95.5% yield, 80% purity).
Yield:72791-76-5 95.5%
Reaction Conditions:
Stage #1: β,β'-dichlordiethylamine hydrochloride salt;benzyl carbonochloridate in dichloromethane at 0; for 0.166667 h;
Stage #2: with triethylamine in dichloromethane at 20; for 2 h;
Steps:
19 N,N-Bis(2-chloroethyl)benzyl carbamate
To a suspension of bis(2-chloroethyl)amine hydrochloride salt (5 g, 0.028 mole) in 30 ml dry dichloro-methane, with cooling (ice water) and stirring, benzyl chloroformate (5.1 g, 0.03 mole) was added dropwise. The addition was complete within 10 min and was followed by addition of triethylamine (6.46 g, 0.063 mole) during 1 h. The mixture was further stirred at room temperature for 1 hour. Water (10 ml) was added, and the mixture was stirred for 15 min. Separated dichchloromethane layer was washed with HCl 1M (10 ml), brine (10 ml), dried and concentrated in vacuo to give an oily product (7 g, 95.5% yield, 80% pur).
References:
US2009/143582,2009,A1 Location in patent:Page/Page column 8