
N-BOC-(S)-2-AMINO-3-HYDROXY-3-METHYLBUTANOIC ACID synthesis
- Product Name:N-BOC-(S)-2-AMINO-3-HYDROXY-3-METHYLBUTANOIC ACID
- CAS Number:102507-13-1
- Molecular formula:C10H19NO5
- Molecular Weight:233.26
![Carbamic acid, [(1R)-2-hydroxy-1-(hydroxymethyl)-2-methylpropyl]-, 1,1-](/CAS/20180713/GIF/473545-40-3.gif)
473545-40-3

102507-13-1
The general procedure for the synthesis of (S)-2-((tert-butoxycarbonyl)amino)-3-hydroxy-3-methylbutanoic acid from tert-butyl (R)-(1,3-dihydroxy-3-methylbutan-2-yl)carbamate was as follows: 2,2,6,6-tetramethylpiperidinium 1-yloxy (TEMPO, 2.3 g, 15 mmol) was added to (R)-(1,3-dihydroxy-3-methyl tert-butyl (33.0 g, 150 mmol) carbamate in acetonitrile (750 mL) and sodium phosphate buffer (600 mL, 0.7 M, pH 6-7) and heated to 35 °C. Subsequently, sodium chlorite solution (34.2 g, dissolved in 150 mL of water) and dilute sodium hypochlorite solution (3 mL of commercial solution diluted in 100 mL of water, 60 drops total) were added dropwise simultaneously to the reaction mixture. The reaction mixture was stirred at 35°C overnight, cooled to room temperature, then the pH was adjusted to 3 with citric acid (~15 g), saturated with sodium chloride, and extracted with ethyl acetate (3 x 2 L). The organic phases were combined and concentrated under reduced pressure. The residue was dissolved in 1.5 L of 2 M sodium carbonate solution and washed with ethyl acetate (2 x 2 L). The aqueous layer was cooled to 0°C, the pH was adjusted to 3.0 with 2M phosphoric acid solution and saturated again with sodium chloride. Extracted with ethyl acetate (3×2L), the organic phases were combined, dried, filtered and concentrated under reduced pressure to give (S)-2-((tert-butoxycarbonyl)amino)-3-hydroxy-3-methylbutanoic acid (28.4 g, 81% yield) as a colorless solid. NMR (400 MHz, DMSO-d6): δ= 1.15 (s, 3H), 1.17 (s, 3H), 1.39 (s, 9H), 3.86 (d, J = 8.6 Hz, 1H), 6.52 (d, J = 8.9 Hz, 1H).
![Carbamic acid, [2-hydroxy-1-(hydroxymethyl)-2-methylpropyl]-, 1,1-](/CAS/20180808/GIF/182958-73-2.gif)
182958-73-2
4 suppliers
inquiry

102507-13-1
134 suppliers
$43.00/100mg
Yield:102507-13-1 76%
Reaction Conditions:
with sodium dihydrogenphosphate in tetrahydrofuran at 0; for 4 h;
Steps:
1.2 Step 2: Preparation of (s)-2-tert-butoxyamido-3-methyl-3-hydroxybutyric acid
(s)-2-tert-butoxyamido-3-methyl-3-hydroxybutanol (20 g) was added to THF (50 mL). After adding saturated sodium dihydrogen phosphate solution (50 mL), the reaction was cooled to 0°C. Add bleach (6%, 350 mL) slowly. After 4 hours of reaction, slowly rise to room temperature. After TLC detects that the reaction is complete, it is quenched by adding sodium thiosulfate. Rotary evaporation to remove THF, then add dichloromethane (100mLX4) for extraction, dry and filter, concentrate to the crude reaction product, add n-hexane (100mL), beating and filtering to obtain pure product (s)-2-tert-butoxyamide -3-Methyl-3-hydroxybutyric acid (16 g, 76%).
References:
Zhejiang Kaipu Chemical Co., Ltd.;Luo Linfeng;Tan Xianghui;Yan Xiaodong;Shen Yipeng;Zhou Chen CN111909067, 2020, A Location in patent:Paragraph 0023-0025; 0028-0029
![Carbamic acid, [(1R)-2-hydroxy-1-(hydroxymethyl)-2-methylpropyl]-, 1,1-](/CAS/20180713/GIF/473545-40-3.gif)
473545-40-3
25 suppliers
$45.00/10mg

102507-13-1
134 suppliers
$43.00/100mg
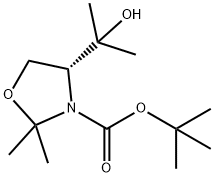
288159-36-4
1 suppliers
inquiry

102507-13-1
134 suppliers
$43.00/100mg
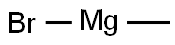
75-16-1
278 suppliers
$12.00/10ml
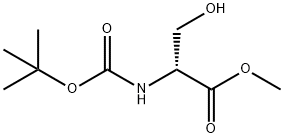
95715-85-8
243 suppliers
$5.00/1g

102507-13-1
134 suppliers
$43.00/100mg
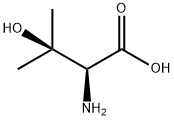
2280-27-5
104 suppliers
$77.00/100mg
34619-03-9
29 suppliers
inquiry

102507-13-1
134 suppliers
$43.00/100mg