
N-Boc-N-methylethylenediamine synthesis
- Product Name:N-Boc-N-methylethylenediamine
- CAS Number:121492-06-6
- Molecular formula:C8H18N2O2
- Molecular Weight:174.24

24424-99-5

109-81-9

121492-06-6
Step 1: N-methylethylenediamine (11.8 mL, 134.9 mmol) was dissolved in acetonitrile (300 mL) and cooled to -30 °C. Subsequently, triethylamine (TEA, 7.46 mL, 53.9 mmol) was added to this solution, followed by dropwise addition of di-tert-butyl dicarbonate (Boc2O, 9.81 g, 45 mmol) in acetonitrile solution. The reaction mixture was stirred at room temperature for 2 hours. After completion of the reaction, the insoluble material was removed by Celite filtration. The filtrate was purified by silica gel column chromatography (elution gradient: 1:50→1:20→1:10 ethyl acetate/hexane) to afford tert-butyl N-(2-aminoethyl)-N-methylcarbamate (CXVIII) as a yellow oil (5.2 g, 29.9 mmol, 66% yield). The product was confirmed by electrospray ionization mass spectrometry (ESIMS) with molecular formula C8H18N2O2, m/z 175 (M + H).
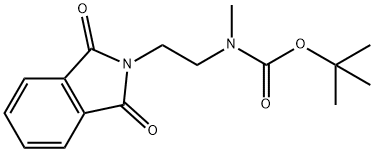
121495-40-7
6 suppliers
inquiry

121492-06-6
223 suppliers
$16.00/1g
Yield:121492-06-6 700 mg
Reaction Conditions:
with hydrazine hydrate in methanol for 5 h;Reflux;
Steps:
10 tert-Butyl (2-ami noethyl)(methyl)carbamate
tert-Butyl (2-ami noethyl)(methyl)carbamate Hydrazine hydrate (400, 8 mmol) was added dropwise to a solution of tert-butyl (2- (i,3-dioxoisoindolin-2-yl)ethyl)(methyl)carbamate (i.2 g, 4 mmol) in MeOH (40 mL). Thenthe mixture was stirred at reflux for S h. After cooling to RT, the solution was filtered to remove insoluble materials and the filtrate was concentrated to dryness under reduced pressure to give an oily residue, to which was added CH2CI2 (iOO mL), and then washed with brine (15 ml). The organic solvent was dried (Na2504) and concentrated to dryness to give tert-butyl (2-aminoethyl)(methyl)carbamate (700 mg) as a colorless oil, which wascarried through without further purification.
References:
TEMPERO PHARMACEUTICALS, INC.;GHOSH, Shomir;LOBERA, Mercedes;SCHMIDT, Darby, R.;BALOGLU, Erkan WO2013/6408, 2013, A1 Location in patent:Page/Page column 53

24424-99-5
866 suppliers
$13.50/25G

109-81-9
147 suppliers
$15.00/1 g

121492-06-6
223 suppliers
$16.00/1g
180976-09-4
52 suppliers
inquiry

121492-06-6
223 suppliers
$16.00/1g

847259-90-9
3 suppliers
inquiry

121492-06-6
223 suppliers
$16.00/1g

181761-60-4
4 suppliers
inquiry

121492-06-6
223 suppliers
$16.00/1g