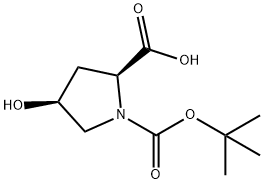
N-Boc-cis-4-Hydroxy-L-proline synthesis
- Product Name:N-Boc-cis-4-Hydroxy-L-proline
- CAS Number:87691-27-8
- Molecular formula:C10H17NO5
- Molecular Weight:231.25

84348-37-8

87691-27-8
General procedure for the synthesis of N-Boc-cis-4-hydroxy-L-proline from (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid: An appropriate amount of solvent was added to the reaction vessel and cooled to the specified temperature. Subsequently, the reducing agent (4.0~5.0 equivalents) was added in batches, taking care to control the rate of addition to avoid violent exotherm. The progress of the reaction was monitored by HPLC during the reaction. Upon completion of the reaction, the reaction solution was slowly poured into ice water to maintain the temperature at 0~5°C. The pH was adjusted to 0~5°C with dilute hydrochloric acid. The pH was adjusted to 2~3 with dilute hydrochloric acid and then extracted with ethyl acetate (EA) (5X each time, 3 times). All organic phases were combined, washed with saturated brine and dried with anhydrous sodium sulfate. The dried organic phase was concentrated under reduced pressure to give the crude product. Finally, the crude product was recrystallized in a mixed solvent of ethyl acetate and alkanes to give purified N-Boc-cis-4-hydroxy-L-proline.

84348-37-8
284 suppliers
$5.00/250mg

87691-27-8
233 suppliers
$6.00/250mg
Yield:87691-27-8 99.1%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0 - 5;
Steps:
3.b Preparation of product cis-F-1
The reducing agent (4.0 to 5.0 e.q.) was added to the solvent,Cold to a certain temperature,Add the substrate in batches to exotherm,HPLC monitoring reaction,Reaction is completed,The reaction solution was added to water,Cooling to 0 ~ 5C,With dilute hydrochloric acid pH 2 ~ 3, EA extraction(5X * 3). The combined organic phases were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give the crude product. The crude productThe product was crystallized from a mixed solvent of ethyl acetate and alkane.
References:
CN106543062,2017,A Location in patent:Paragraph 0021; 0024; 0027

24424-99-5
865 suppliers
$13.50/25G
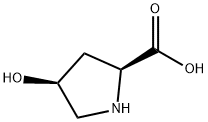
618-27-9
313 suppliers
$9.00/250mg

87691-27-8
233 suppliers
$6.00/250mg
168264-25-3
8 suppliers
inquiry

87691-27-8
233 suppliers
$6.00/250mg

13726-69-7
436 suppliers
$5.00/1g

87691-27-8
233 suppliers
$6.00/250mg
![tert-butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/GIF/87250-97-3.gif)
87250-97-3
10 suppliers
$350.00/1g

87691-27-8
233 suppliers
$6.00/250mg