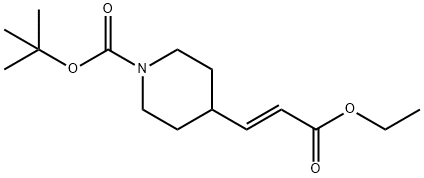
N-BOC-4-(2-ETHOXYCARBONYL-VINYL)-PIPERIDINE synthesis
- Product Name:N-BOC-4-(2-ETHOXYCARBONYL-VINYL)-PIPERIDINE
- CAS Number:198895-61-3
- Molecular formula:C15H25NO4
- Molecular Weight:283.36
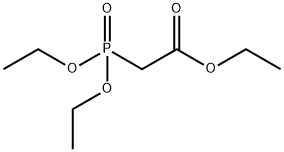
867-13-0
475 suppliers
$10.00/1g

137076-22-3
351 suppliers
$5.00/1g

198895-61-3
24 suppliers
$295.00/1g
Yield:198895-61-3 70%
Reaction Conditions:
Stage #1: diethoxyphosphoryl-acetic acid ethyl esterwith sodium hydride in tetrahydrofuran at 0; for 0.5 h;Inert atmosphere;
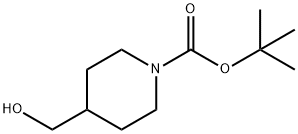
Stage #2: 4-formyl-piperidine-1-carboxylic acid tert-butyl ester in tetrahydrofuran at 25; for 14 h;Inert atmosphere;
Steps:
26 Synthesis of tert-butyl 4-[3-ethoxy-3-oxoprop-1-en-1-yl]piperidine-1 -carboxylate:
To a stirred solution of triethyl phosphonoacetate (15.8 g, 70.3 mmol, 1.0 eq) in tetrahydrofuran was added NaH (2.8 g, 70.3 mmol, 1 .0 eq) dropwiseat 0°C under N2 atmosphere. The reaction mixture was stirred for 30 min at 0°C. After that, to the above mixture was added tert-butyl 4-formylpiperidine-1 -carboxylate (15 g, 70.3 mmol, 1.0 eq). The reaction mixture was stirred for 14 hours at 25°C. The resulting mixture was quenched by the addition of water (5 mL) at 0°C and then concentrated under vacuum. The residue was diluted with dichloromethane (200 mL), washed with water (50 mL) and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under vacuum. The crude residue was purified by a flash column (silica gel, ethyl acetate/petroleum ether=0:1 to 10:1) to give tert-butyl 4-[3-ethoxy-3-oxoprop-1 -en-1 -yl]piperidine-1 -carboxylate (14.0 g, 70 %) as a yellow oil. 1HNMR (300 MHz, DMSO-d6) 5 6.85 (dd, J=15.9, 6.6 Hz, 1 H), 5.89-5.73 (m, 1 H), 4.11 (q, J=7.2 Hz, 2H), 3.95 (d, J=13.2 Hz, 2H), 2.75 (s, 2H), 2.43-2.27 (m, 1H), 1.74-1.58 (m, 2H), 1.40 (s, 9H), 1.30-1.11 (m, 5H).
References:
WO2022/159644,2022,A1 Location in patent:Page/Page column 130
123855-51-6
412 suppliers
$6.00/5g

198895-61-3
24 suppliers
$295.00/1g
1099-45-2
371 suppliers
$6.00/5g

137076-22-3
351 suppliers
$5.00/1g

198895-61-3
24 suppliers
$295.00/1g