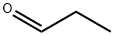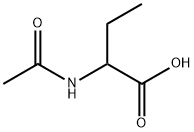
N-ACETYL-DL-2-AMINO-N-BUTYRIC ACID synthesis
- Product Name:N-ACETYL-DL-2-AMINO-N-BUTYRIC ACID
- CAS Number:7211-57-6
- Molecular formula:C6H11NO3
- Molecular Weight:145.16
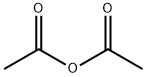
108-24-7
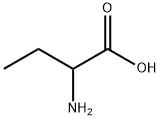
2835-81-6

7211-57-6
General procedure for the synthesis of 2-acetylaminobutyric acid from ethanoic anhydride and DL-2-aminobutyric acid: 1. dissolve 163 g (1.58 mol) of DL-2-aminobutyric acid in an appropriate amount of acetic acid. 2. 242 g (2.37 mol) of acetic anhydride was added slowly and dropwise to the above solution. 3. the reaction mixture was stirred continuously at 100 °C for 2 hours until the reaction was complete. 4. After completion of the reaction, the solvent was removed by vacuum evaporation to give a solid residue. 5. The solid residue was suspended in ethyl acetate and filtered. 6. The filter cake was washed with ether to remove unreacted raw materials and by-products. 7. The target product 2-acetylaminobutyric acid was obtained after drying in a yield of 220 g (95.9% yield). Product characterization data: 1H-NMR (methanol-d4) δ/ppm 0.97 (t, 3H), 1.65-1.93 (m, 2H), 1.99 (s, 3H), 4.29 (q, 1H).

2835-81-6
366 suppliers
$5.00/5g

7211-57-6
55 suppliers
$14.00/1g
Yield:7211-57-6 220 g (96%)
Reaction Conditions:
with acetic anhydride in acetic acid;ethyl acetate;
Steps:
1.A 2-(Acetylamino)butanoic Acid
EXAMPLE 1A 2-(Acetylamino)butanoic Acid 163 g (1,58 mol) 2-aminobutanoic acid are dissolved in acetic acid, and 242 g (2,37 mol) acetic anhydride are added dropwise. The mixture is stirred for 2 h at 100° C. until completion of reaction, then the solution evaporated to dryness in vacuo. The solid residue is suspended in ethyl acetate, filtered and washed with diethyl ether. Yield: 220 g (96%) 1H-NMR (Methanol-d4): δ=0,97 (t, 3H), 1,65-1,93 (m, 2H), 1,99 (s, 3H), 4,29 (q, 1H) ppm.
References:
US2003/139415,2003,A1
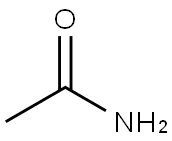
60-35-5
527 suppliers
$13.00/25g
201230-82-2
1 suppliers
inquiry

107-18-6
0 suppliers
$29.19/5ML

7211-57-6
55 suppliers
$14.00/1g
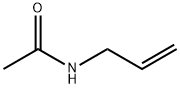
692-33-1
15 suppliers
inquiry

7211-57-6
55 suppliers
$14.00/1g