N-(4-CYANO-PHENYL)-GLYCINE synthesis
- Product Name:N-(4-CYANO-PHENYL)-GLYCINE
- CAS Number:42288-26-6
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
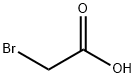
79-08-3

873-74-5

42288-26-6
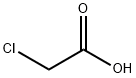
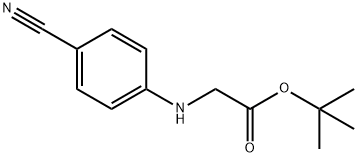
3.2.3 Preparation of [(4-cyanophenyl)amino]acetic acid (V). Molecular formula: C9H8N2O2. molecular weight: 176.17. Raw materials: 90g (0.75mol) of bromoacetic acid (F), 211.7g (1.5mol) of p-aminobenzonitrile (G), 35g (0.42mol) of sodium bicarbonate. Procedure: bromoacetic acid (F) and p-aminobenzonitrile (G) were mixed in 1250 ml of water to form a suspension. The suspension was placed in a bath heated to 100-110°C for 3 hours. Upon completion of the reaction, the reaction vessel was removed from the bath and cooled to room temperature, followed by further cooling in a refrigerator. The precipitate was separated by diafiltration and dried in a vacuum desiccator at 100 °C. Yield of crude product: 122 g (92.8% yield), HPLC purity: 97%. Purification step: the crude product was converted to sodium salt and re-acidified using aqueous sodium bicarbonate to release the free acid by dilute hydrochloric acid (1:1). After filtration, the product was washed with water and dried in a vacuum desiccator at 105°C. The product was then purified to a sodium salt using aqueous sodium bicarbonate. Yield of purified product: 115 g (88% yield), HPLC purity: 99.1%, water content: 0.13%, sulfated ash: 1.8%.
Yield:42288-26-6 88%
Reaction Conditions:
in water at 100 - 110; for 3 h;
Steps:
3
3.2.3 Preparation of [(4-cyanophenyl)amino]acetic acid (V) C7H6N2 C2H3DPθ2 C9H8N2O2 MoI. Wt.: 1 18,14 MoI. Wt.: 138,95 MoI. Wt.: 176,17VIngredientsF: 90 g - 0.75 molG: 211.7 g - 1.5 molSodium bicarbonate: 35 g, 0.42 molThe starting substances F and G were mixed in 1250 ml of water and this suspension was inserted into a bath heated up to 100-110 0C. After three hours of heating the reaction container was removed from the bath, cooled in the fridge and the separated substance was sucked off. The substance was dried in a vacuum drier at the temperature of 100 °C. Yield: Crude product: 122 g (92.8%), HPLC: 97%The crude product was purified by conversion to the sodium salt and re-acidification using an aqueous solution of sodium bicarbonate. The acid was released by means of diluted hydrochloric acid (1 :1). After sucking off and washing with water the product was dried in a vacuum drier (105 °C). Yield: Purified product: 115 g (88%), HPLC: 99.1%, water content: 0.13%; sulphate ash: 1.8%
References:
ZENTIVA, K.S. WO2009/111997, 2009, A1 Location in patent:Page/Page column 11
3926-62-3
299 suppliers
$16.69/250g

873-74-5
660 suppliers
$9.00/1g

42288-26-6
560 suppliers
$14.00/1g
623-00-7
486 suppliers
$6.00/10g
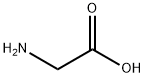
56-40-6
1315 suppliers
$5.00/25g

42288-26-6
560 suppliers
$14.00/1g
144656-11-1
3 suppliers
inquiry

42288-26-6
560 suppliers
$14.00/1g