
N-(2-Bromophenyl)methansulfonamide synthesis
- Product Name:N-(2-Bromophenyl)methansulfonamide
- CAS Number:116547-91-2
- Molecular formula:C7H8BrNO2S
- Molecular Weight:250.11
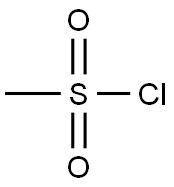
124-63-0
0 suppliers
$10.00/5g
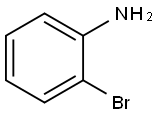
615-36-1
418 suppliers
$10.00/5g

116547-91-2
23 suppliers
$45.00/10mg
Yield:116547-91-2 96%
Reaction Conditions:
with pyridine in acetonitrile at 0 - 20; for 0.5 h;
Steps:
1 Preparation of compound 1-e.
2-Bromoaniline (10.0 g, 58.5 mmol) was dissolved in pyridine (50 mL) and acetonitrile (50 mL). The reactionsolution was cooled to 0°C and methanesulfonyl chloride (10.0 g, 87.7 mmol) was added dropwise. The reaction mixturewas allowed to warm to room temperature and the stirring was continued for 30 minutes and then concentrated underreduced pressure. The residue was dissolved in ethyl acetate (250 mL) and diluted with water (250 mL). The pH of theseparated organic phase was adjusted to 7 with 1 M aqueous hydrochloric acid solution. The organic phase was driedover anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to deliver a yellowsolid 1-e (14 g, yield: 96%). This product was used without further purification. LC-MS (ESI): m/z = 250 [M+H] +.
References:
EP3287463,2018,A1 Location in patent:Paragraph 0104; 0109
67-68-5
1298 suppliers
$15.00/100g

615-36-1
418 suppliers
$10.00/5g

116547-91-2
23 suppliers
$45.00/10mg