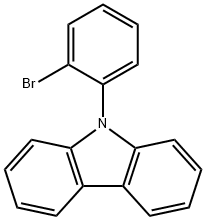
N-(2-BroMophenyl)-9H-carbazole synthesis
- Product Name:N-(2-BroMophenyl)-9H-carbazole
- CAS Number:902518-11-0
- Molecular formula:C18H12BrN
- Molecular Weight:322.2
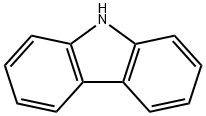
86-74-8
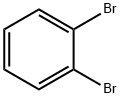
583-53-9

902518-11-0
(1) Carbazole (8.36 g, 0.05 mol), 1,2-dibromobenzene (17.69 g, 0.075 mol) and potassium carbonate (17.97 g, 0.13 mol) were added to a reactor under the protection of nitrogen and stirred thoroughly. Subsequently, cuprous iodide (1.91 g) and L-lysine (1.46 g) were added to the system, heated to 163 °C and maintained for 38 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) until the feedstock was completely consumed. Upon completion of the reaction, the reaction was quenched by the addition of large amounts of water and the solid product was precipitated by stirring. The solid was collected by filtration and dried to give 13.37 g of 9-(2'-bromophenyl)carbazole in 83% yield.
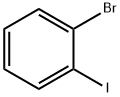
583-55-1
480 suppliers
$7.00/5g

86-74-8
347 suppliers
$10.00/5g

902518-11-0
185 suppliers
$9.00/250mg
Yield:902518-11-0 96%
Reaction Conditions:
with tripotassium phosphate tribasic;copper (I) iodide;(±)-trans-1,2-diaminocyclohexane in toluene; for 4 h;
Steps:
A1-1 Synthesis of Intermediate A(1)-P3
Carbazole (40 g, 1 eq.), 1-bromo-2-iodobenzene (70 g, 1.5 eq.), CuI (6.3 g, 0.2 eq.), K3PO4 (70 g, 2 eq.), trans-1,2-cyclohexanediamine (19 g, 1 eq.) and toluene (600 ml) were introduced to a 1-neck-round bottom flask (1-neck-r.b.f), and stirred for 4 hours. (0137) The reaction solution completed with the stirring was filtered only with methylene chloride (MC), and then column separated to obtain P3 (approximately 65 g). (0138) Step yield=96%
References:
US2022/106332,2022,A1 Location in patent:Paragraph 0135-0138
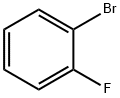
1072-85-1
460 suppliers
$5.00/10g

86-74-8
347 suppliers
$10.00/5g

902518-11-0
185 suppliers
$9.00/250mg

86-74-8
347 suppliers
$10.00/5g

583-53-9
325 suppliers
$10.00/5g

902518-11-0
185 suppliers
$9.00/250mg

86-74-8
347 suppliers
$10.00/5g

16732-66-4
54 suppliers
inquiry

902518-11-0
185 suppliers
$9.00/250mg

86-74-8
347 suppliers
$10.00/5g

583-53-9
325 suppliers
$10.00/5g

1150-62-5
291 suppliers
$8.00/5g

902518-11-0
185 suppliers
$9.00/250mg