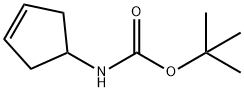
N-1-Boc-amino-3-cyclopentene synthesis
- Product Name:N-1-Boc-amino-3-cyclopentene
- CAS Number:193751-54-1
- Molecular formula:C10H17NO2
- Molecular Weight:183.25
![Acetic acid, 2-[3-cyclopenten-1-yl[(1,1-dimethylethoxy)carbonyl]amino]-2-oxo-, ethyl ester](/CAS/20210305/GIF/1018908-86-5.gif)
1018908-86-5

193751-54-1
GENERAL STEPS: To a three-necked round-bottomed flask cooled in an ice-water bath was added an anhydrous tetrahydrofuran (THF, 10 mL) solution of triphenylphosphine (Ph3P, 0.015 mol), followed by an anhydrous THF (10 mL) solution of 3-cyclopenten-1-ol (II, 0.012 mol) and an anhydrous THF (0.015 mol) solution of N-Boc-ethyl oxalate ( 10 mL) solution. Diethyl azodicarboxylate (DEAD, 0.015 mol) was slowly added dropwise to the above mixture. The reaction mixture was stirred at 0°C for 2 hours, followed by continuing the reaction at room temperature for 48 hours. Upon completion of the reaction, the solvent was removed by reduced pressure distillation and the residue was dissolved in dichloromethane (CH2Cl2, 30 mL) and washed sequentially with water and brine (20 mL x 3). The solvent was again removed by reduced pressure distillation and the residue was purified by column chromatography (silica gel, 100% dichloromethane) to afford a mixture of ethyl (tert-butoxycarbonyl-cyclopent-3-enyl-amino)-oxo-acetate and N-Boc-ethylcarbamate, which was used for the next reaction without further purification. The above crude product (3.80 g) was dissolved in THF (35 mL), cooled in an ice-water bath and stirred, and a solution of lithium hydroxide (LiOH, 0.0765 mol) in water (35 mL) was slowly added. The mixture was continued to be stirred in an ice-water bath for 3 hours. Upon completion of the reaction, the organic material was extracted with dichloromethane (30 mL x 3), the organic layers were combined and washed with brine (30 mL x 2), and the solvent was subsequently removed by distillation under reduced pressure. The residue was purified by column chromatography (silica gel, 100% dichloromethane) to give white crystals of the pure product in 53% yield.
24424-99-5
867 suppliers
$13.50/25G

91469-55-5
106 suppliers
$17.00/100mg

193751-54-1
114 suppliers
$33.00/250mg
Yield:193751-54-1 85%
Reaction Conditions:
with triethylamine in dichloromethane at 18; for 10 h;Inert atmosphere;
Steps:
8.1 The first step: the preparation of tert-butyl cyclopent-3-en-1-ylcarbamate:
Compound 3-cyclopentenylamine hydrochloride (4.5g, 37.625mmol, 1.0eq) was dissolved in dichloromethane (188ml), under nitrogen protection, di-tert-butyl dicarbonate (9.85g, 45.151mmol, 1.2eq) and compound triethanolamine (23mL, 165.552mmol, 4.4eq), the reaction solution was reacted at room temperature (18°C) overnight (10h). Detection (TLC and LCMS) after the completion of the reaction, add water (30 mL), extract three times with dichloromethane, each 100 mL, dry over anhydrous sodium sulfate, filter, concentrate, and the crude product is subjected to column chromatography (petroleum ether: ethyl acetate=5 : 1) The intermediate cyclopent-3-en-1-ylcarbamate tert-butyl ester 5.85 g was isolated and obtained, and the yield was 85.0%.
References:
WO2022/48631,2022,A1 Location in patent:Page/Page column 43-44
![Acetic acid, 2-[3-cyclopenten-1-yl[(1,1-dimethylethoxy)carbonyl]amino]-2-oxo-, ethyl ester](/CAS/20210305/GIF/1018908-86-5.gif)
1018908-86-5
0 suppliers
inquiry

193751-54-1
114 suppliers
$33.00/250mg
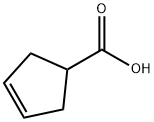
7686-77-3
270 suppliers
$6.00/1g

193751-54-1
114 suppliers
$33.00/250mg

14320-38-8
181 suppliers
$6.00/250mg
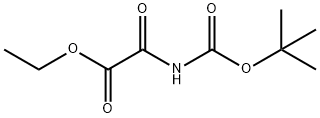
216959-34-1
74 suppliers
$22.00/100mg

193751-54-1
114 suppliers
$33.00/250mg

7686-77-3
270 suppliers
$6.00/1g
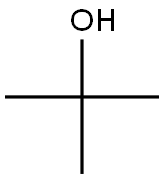
75-65-0
784 suppliers
$10.00/10ml

193751-54-1
114 suppliers
$33.00/250mg