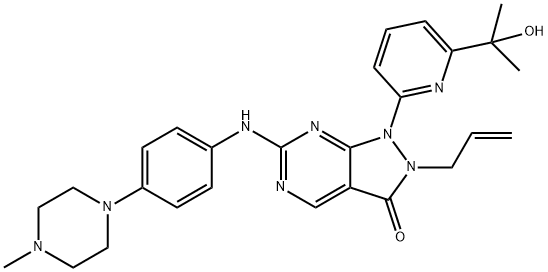
MK-1775 synthesis
- Product Name:MK-1775
- CAS Number:955365-80-7
- Molecular formula:C27H32N8O2
- Molecular Weight:500.6
![2-allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidin-3(2H)-one](/CAS/GIF/955369-56-9.gif)
955369-56-9
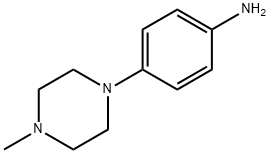
16153-81-4

955365-80-7
Synthesis of 2-allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(methylmercapto)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one and 4-(4-methyl-1-piperazinyl)aniline using 2-allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazin-1- based) phenylamino)-1H-pyrazolo[3,4-D]pyrimidin-3(2H)-one was prepared in the following general procedure: to a 20 mL solution of 1.10 g of 2-allyl-1-(6-(2-hydroxypropan-2- yl)pyridin-2-yl)-6-(methylmercapto)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one in toluene was added 817 mg of isochloroperbenzoic acid ( purity >65%) and the reaction was stirred for 20 minutes. Subsequently, 1.61 mL of N,N-diisopropylethylamine and 706 mg of 4-(4-methylpiperazin-1-yl)aniline were added to the reaction system and the reaction was continued with stirring overnight. After completion of the reaction, saturated aqueous sodium bicarbonate was added to the reaction solution, the organic phase was extracted with ethyl acetate, then the organic phase was washed with saturated brine and finally dried with anhydrous magnesium sulfate. The solvent was removed by distillation under reduced pressure, and the resulting residue was purified by basic silica gel column chromatography (eluent ratio: hexane/ethyl acetate=1/1~0/1, followed by ethyl acetate/ethanol=98/2). The purified solution was concentrated and recrystallized with ethyl acetate to afford 1.20 g of the target compound 2-allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazin-1-yl)phenylamino)-1H-pyrazolo[3,4-D]pyrimidin-3(2H)-one as a yellow solid. The product was confirmed by 1H-NMR (400 MHz, CDCl3) and ESI-MS (m/z [M+H]+ 501) characterization.
26218-75-7
241 suppliers
$9.00/1g

955365-80-7
230 suppliers
$28.00/1unit
Yield:-
Steps:
Multi-step reaction with 3 steps
1.1: diethyl ether / 0.08 h / 20 °C / Inert atmosphere
2.1: potassium carbonate; N,N`-dimethylethylenediamine; copper(l) iodide / 1,4-dioxane / 18 h / 80 - 95 °C
3.1: 3-chloro-benzenecarboperoxoic acid / toluene / 1 h / 20 °C
3.2: 18 h / 20 °C
References:
Matheson, Christopher J.;Casalvieri, Kimberly A.;Backos, Donald S.;Reigan, Philip [ChemMedChem,2018,vol. 13,# 16,p. 1681 - 1694]
![2-allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidin-3(2H)-one](/CAS/GIF/955369-56-9.gif)
955369-56-9
42 suppliers
$287.00/100mg

16153-81-4
304 suppliers
$9.00/250mg

955365-80-7
230 suppliers
$28.00/1unit

16153-81-4
304 suppliers
$9.00/250mg

955365-80-7
230 suppliers
$28.00/1unit
638218-78-7
103 suppliers
$37.00/100mg

955365-80-7
230 suppliers
$28.00/1unit
![2-allyl-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidin-3(2H)-one](/CAS/GIF/955368-90-8.gif)
955368-90-8
72 suppliers
$25.00/100mg

955365-80-7
230 suppliers
$28.00/1unit