
Methyl L-prolinate hydrochloride synthesis
- Product Name:Methyl L-prolinate hydrochloride
- CAS Number:2133-40-6
- Molecular formula:C6H12ClNO2
- Molecular Weight:165.62

67-56-1
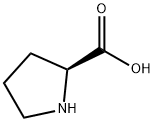
147-85-3

2133-40-6
The general procedure for the synthesis of L-proline methyl ester hydrochloride from methanol and L-proline was as follows: first, methyl (S)-pyrrolidine-2-carboxylate was prepared by reacting (S)-proline (10.2 g, 88.6 mmol) with SOCl? (11.6 g, 7.10 mL, 97.5 mmol) in refluxed methanol (60 mL) for 29 hours. Upon completion of the reaction, excess solvent and SOCl? were removed by distillation under reduced pressure to afford the proline ester as a gray oil (15.9 g, quantitative yield). Subsequently, 2-fluoro-1-nitrobenzene (6.92 g, 5.18 mL, 49.0 mmol) was reacted with methyl (S)-pyrrolidine-2-carboxylate (7.55 g, 45.6 mmol) prepared as described above according to the literature method to afford bright yellow crystals of L-prolinylmethyl ester hydrochloride (12.0 g, quantitative yield), which was used without further purification for subsequent The product can be used for subsequent experiments without further purification.

67-56-1
782 suppliers
$9.00/25ml
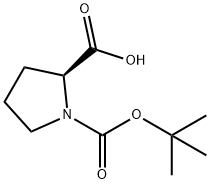
15761-39-4
618 suppliers
$5.00/10g

2133-40-6
448 suppliers
$8.00/5g
Yield:2133-40-6 88%
Reaction Conditions:
Stage #1: methanol;1-(tert-butoxycarbonyl)-L-prolinewith thionyl chloride at 70; for 7.5 h;Inert atmosphere;Cooling with ice;
Stage #2: with hydrogenchloride in dichloromethane;ethyl acetate at 20; for 0.0833333 h;Inert atmosphere;
Steps:
20.1 Step 1) : (S) -methyl pyrrolidine-2-carboxylate hydrochloride
To a solution of (S) -1- (tert-butoxycarbonyl) pyrrolidine-2-carboxylic acid (1.50 g, 6.97 mmol) in methanol (20 mL) was added sulfoxide chloride (0.51 mL, 6.97 mmol) dropwise in an ice-bath. The mixture was stirred in the ice-bath for 30 min, and then stirred at 70 for 7 h. The reaction mixture was concentrated, and DCM (8 mL) and a HCl in EtOAc solution (4 M, 6 mL) were added. The resulting mixture was stirred at rt for 5 min, and then concentrated to give the title compound as white thick oil (800 mg, 88) .1H NMR (400 MHz, CD3OD) : δ ppm 4.47 (t, J 7.8 Hz, 1H) , 3.88 (s, 3H) , 3.37-3.45 (m, 2H) , 2.41-2.50 (m, 1H) , 2.07-2.21 (m, 3H) .
References:
WO2016/34134,2016,A1 Location in patent:Paragraph 00324
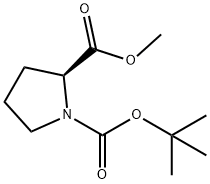
59936-29-7
232 suppliers
$6.00/5g

2133-40-6
448 suppliers
$8.00/5g

5211-23-4
63 suppliers
$7.00/1g

2133-40-6
448 suppliers
$8.00/5g

147-85-3
1064 suppliers
$6.00/25g

2133-40-6
448 suppliers
$8.00/5g