Methyl L-leucinate hydrochloride synthesis
- Product Name:Methyl L-leucinate hydrochloride
- CAS Number:7517-19-3
- Molecular formula:C7H16ClNO2
- Molecular Weight:181.66

67-56-1
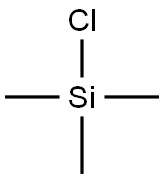
61-90-5

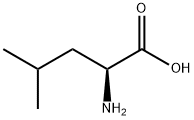
7517-19-3
Under ice bath conditions, 60 mL of methanol was added to a 100 mL round bottom flask. 4 mL of sulfoxide chloride (SOCl2) was added slowly and dropwise through a constant pressure dropping funnel (with a drying tube fitted at the top), while the resulting off-gas was absorbed using NaOH solution. After stirring the reaction mixture for 1 h, 8 mmol of L-leucine was added and stirring was continued for 30 min at room temperature. Subsequently, the reaction system was warmed up to 66 °C and the reaction was refluxed for 6 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) with an ethanol solution of 2% ninhydrin as the color developer until the raw material spot disappeared. After completion of the reaction, the solvent was removed by distillation under reduced pressure to give L-leucine methyl ester hydrochloride in 100% yield.

61-90-5
858 suppliers
$10.00/25G

7517-19-3
363 suppliers
$5.00/5g
Yield:7517-19-3 98%
Reaction Conditions:
with thionyl chloride in methanol
Steps:
179.1 Trans-4-Acetylthio-5-isobutyl-1-(4-phenoxybenzyl)pyrrolidin-2-one
(1) Similarly to Example 20 and starting from 6.56 g (50.0 mmol) of D,L-leucine, 50 ml of methanol and 13.0 ml (180 mmol) of thionyl chloride, 8.93 g (yield: 98%) of D,L-leucine methyl ester hydrochloride was obtained as a white powder. 1H-NMR (300 MHz, D2O); δ: 4.17 (1H, t, J=6.8 Hz), 3.87 (3H, s), 1.90 (1H, m), 1.78 (2H, m), 00 (3H, d, J=6.0 Hz), 0.99 (3H, d, J=6.0 Hz).
References:
Takeda Chemical Industries, Ltd. US6420415, 2002, B1

67-56-1
781 suppliers
$7.29/5ml-f
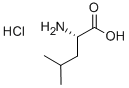
760-84-9
23 suppliers
$63.20/5ml-f

7517-19-3
363 suppliers
$5.00/5g

67-56-1
781 suppliers
$7.29/5ml-f
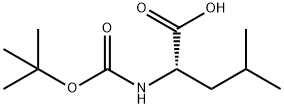
13139-15-6
447 suppliers
$7.00/5g

7517-19-3
363 suppliers
$5.00/5g