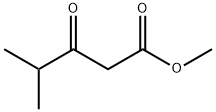
Methyl isobutyrylacetate synthesis
- Product Name:Methyl isobutyrylacetate
- CAS Number:42558-54-3
- Molecular formula:C7H12O3
- Molecular Weight:144.17

563-80-4
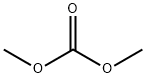
616-38-6

42558-54-3
The general procedure for the synthesis of methyl isobutyrylacetate from 3-methyl-2-butanone and dimethyl carbonate was as follows: first, sodium hydride (70.32 g, 2.1 eq., 50% dispersion in oil) was washed with toluene, and the washings were decanted. Next, anhydrous toluene (500 mL) and dimethyl carbonate (329.3 g, 2 eq.) were added to the reaction system and the stirred mixture was heated to 80 °C under nitrogen protection. Meanwhile, 3-methyl-2-butanone (120 g, 1 eq.) was dissolved in 240 mL of toluene and slowly added to the reaction system. After the reaction lasted for 5 hours, the cooled mixture was poured into a mixture containing glacial acetic acid (300 mL) and water (120 mL). The organic layer was separated and the aqueous layer was further extracted with ethyl acetate (300 mL). All organic extracts were combined and after evaporation of the solvent, the residue was distilled to afford methyl 4-methyl-3-oxopentanoate as a slurry (138 g, 62.72% yield).

563-80-4
316 suppliers
$18.00/25mL

616-38-6
719 suppliers
$5.00/5G
16466-21-0
1 suppliers
inquiry
101459-87-4
0 suppliers
inquiry

42558-54-3
390 suppliers
$10.00/5g
Yield:42558-54-3 88%
Reaction Conditions:
Stage #1: 3-methyl-butan-2-onewith sodium hydride in 1,4-dioxane;mineral oil at 20; for 0.166667 h;
Stage #2: carbonic acid dimethyl ester in 1,4-dioxane;mineral oil at 50; for 3 h;Reagent/catalyst;Solvent;Temperature;Time;
Steps:
Preparation of Methyl 4-Methyl-3-oxopentanoate[4b] (2)
Methyl isopropyl ketone (10.00 g,116.10 mmol) was added slowly to a suspension of sodium hydride (60 % in mineral oil, 9.29 g, 232.25 mmol) in 1,4-dioxane (70 mL). The mixture was stirred at room temperature for 10 min. Dimethyl carbonate (15.70 g, 174.31 mmol) was added and the mixture was stirred at 50 °C for 3 h. The mixture was cooled to room temperature, and acetic acid (14mL) was slowly added. The precipitate was filtered and washed with 1,4-dioxane(20 mL). The filtrate was concentrated unde rvacuum. Compound 2 was collected at 80-85 °C under vacuum (~10 mbar)as colorlessl iquid (14.73g, yield 88 %). 1H NMR (600 MHz,CDCl3) δ: 3.69 (s,3H), 3.48 (s,2H), 2.68 (m,1H), 1.01 (d, J7.2 Hz, 6H). 13C NMR (150 MHz,CDCl3) δ: 205.95, 167.38, 51.44, 46.23, 40.44, 17.26.
References:
Xing, Yuzhi;Chen, Shipeng;Zhou, Yingtao;Liu, Na;Chen, Ligong;Li, Yang [Synthetic Communications,2015,vol. 45,# 24,p. 2832 - 2840]

563-80-4
316 suppliers
$18.00/25mL

616-38-6
719 suppliers
$5.00/5G

42558-54-3
390 suppliers
$10.00/5g
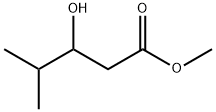
65596-31-8
7 suppliers
inquiry

42558-54-3
390 suppliers
$10.00/5g
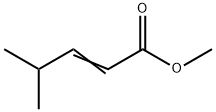
50652-78-3
33 suppliers
$1112.27/10G

42558-54-3
390 suppliers
$10.00/5g

67-56-1
782 suppliers
$9.00/25ml
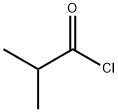
79-30-1
380 suppliers
$10.00/10g
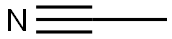
75-05-8
1038 suppliers
$10.00/10g

42558-54-3
390 suppliers
$10.00/5g