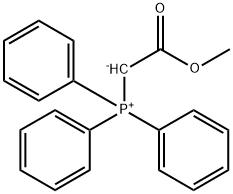
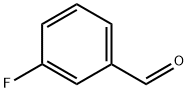
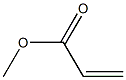
METHYL (E)-3-FLUOROCINNAMATE synthesis
- Product Name:METHYL (E)-3-FLUOROCINNAMATE
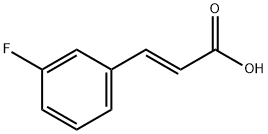
- CAS Number:74325-03-4
- Molecular formula:C10H9FO2
- Molecular Weight:180.18

67-56-1
780 suppliers
$9.00/25ml
20595-30-6
172 suppliers
$6.00/5g

74325-03-4
36 suppliers
$25.00/1g
Yield:74325-03-4 99%
Reaction Conditions:
with sulfuric acid at 0;Reflux;Inert atmosphere;
Steps:
1
Example 1; [0052] (/+/-)-Methyl 3-(3-fluorophenyl)acrylate (6). To a solution of (£)-3-(3- fluorophenyl)acrylic acid (5, 6.0 g, 32.7 mmol) in MeOH (150 mL) at 0 0C was added concentrated H2SO4 (3.0 mL) slowly. The reaction was heated under reflux for 24 h then cooled to room temperature and neutralized with Na2CO3 (1.0 g). The solvent was removed by rotary evaporation and the resulting yellow oil partitioned between EtOAc (300 mL) and saturated NaHCO3 (300 mL). The aqueous layer was extracted with EtOAc (2 x 100 mL). The combined organic layers were washed with brine (200 mL) and dried over Na2SO4. The solvent was removed by rotary evaporation to yield 6 as a low melting (<;25°C) white solid (32.0 mmol, 99%): 1R NMR (500 MHz, CDCl3) δ 3.79 (s, 3H), 6.39-6.43 (d, J= 16.0 Hz, IH), 7.04-7.07 (dt, J= 2.0, 8.0 Hz, IH), 7.18-7.20 (d, J= 9.0 Hz, IH), 7.25-7.26 (d, J= 8.0 Hz, IH), 7.30-7.35 (m, IH), 7.60-7.64 (d, J= 16.0 Hz, IH); 13C NMR (125 MHz, CDCl3) δ 52.0, 114.4, 114.6, 117.3, 117.4, 119.4, 124.29, 124.32, 130.6, 130.7, 136.8, 136.9, 143.6, 143.7, 162.2, 164.2, 167.2; LCQ-MS (M + H+) calcd for C10H10FO2 181, found 181.
References:
WO2010/85749,2010,A2 Location in patent:Page/Page column 22-23

20595-30-6
172 suppliers
$6.00/5g

74-88-4
354 suppliers
$15.00/10g

74325-03-4
36 suppliers
$25.00/1g

20595-30-6
172 suppliers
$6.00/5g

74325-03-4
36 suppliers
$25.00/1g