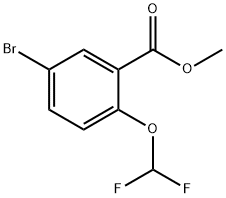
Methyl 5-broMo-2-(difluoroMethoxy)benzoate synthesis
- Product Name:Methyl 5-broMo-2-(difluoroMethoxy)benzoate
- CAS Number:1131587-78-4
- Molecular formula:C9H7BrF2O3
- Molecular Weight:281.05
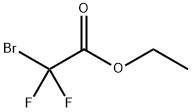
667-27-6
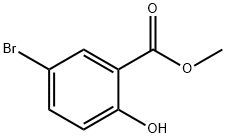
4068-76-2

1131587-78-4
To a solution of methyl 5-bromo-2-hydroxybenzoate (409 mg, 1.77 mmol) in anhydrous DMF (5 mL) was sequentially added potassium carbonate (367 mg, 2.67 mmol) and ethyl 2-bromo-2,2-difluoroacetate (280 μL, 2.13 mmol). The reaction mixture was stirred at 80 °C for 24 hours. After completion of the reaction, the mixture was cooled to room temperature, diluted with ether (10 mL) and washed sequentially with water (10 mL), 10% aqueous hydrochloric acid solution (10 mL), water (10 mL) and saturated saline (10 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (elution gradient: n-hexane to n-hexane/ethyl acetate=2:1, v/v) to afford methyl 5-bromo-2-(difluoromethoxy)benzoate as a yellow oil (250 mg, 50% yield).

667-27-6
421 suppliers
$10.00/10g

4068-76-2
181 suppliers
$8.00/1g

1131587-78-4
55 suppliers
$52.00/250mg
Yield:1131587-78-4 50%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 24 h;
Steps:
121.1
To a solution of methyl 5-bromo-2-hydroxybenzoate (409 mg, 1.77 mmol) in DMF (5 mL) was added potassium carbonate (367 mg, 2.67 mmol) and ethyl 2-bromo-2,2-difluoroacetate (280 μ^, 2.13 mmol). The resulting mixture was heated at 80 °C for 24 hrs. The resulting solution was cooled to RT, diluted with ether and washed sequentially with water, 10% aq. HCl, water and brine. The organic extract was then dried over Na2S04, filtered and the filtrate concentrated in vacuo. Further purification by way of column chromatography (S1O2, gradient elution, Hex to 2:1 (v/v) Hex: EtOAc) afforded methyl 5-bromo-2- (difluoromethoxy)benzoate as a yellow oil (250 mg, 50% yield).
References:
WO2013/134562,2013,A1 Location in patent:Paragraph 00294

4068-76-2
181 suppliers
$8.00/1g
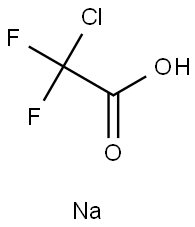
1895-39-2
292 suppliers
$6.00/5g

1131587-78-4
55 suppliers
$52.00/250mg