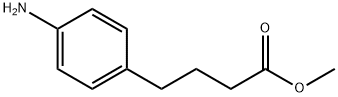
METHYL 4-(4-AMINOPHENYL)BUTANOATE synthesis
- Product Name:METHYL 4-(4-AMINOPHENYL)BUTANOATE
- CAS Number:20637-09-6
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
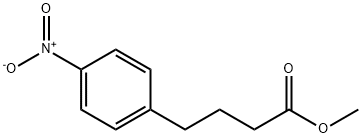
20637-02-9

20637-09-6
b) Synthesis of methyl 4-(4-aminophenyl)butyrate Methyl 4-(4-nitrophenyl)butyrate (3.05 g, 13.67 mmol) and Pd/C catalyst (1.0 g, 10% Pd, activated carbon carrier, 1.09 mmol) were suspended in methanol (40 mL). The reaction mixture was stirred under hydrogen atmosphere (balloon pressure) for 1 hour. After completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad (washed with ethyl acetate). The filtrate was concentrated under reduced pressure to give 2.33 g of methyl 4-(4-aminophenyl)butyrate (brown solid, yield: 88%). The product did not require further purification and could be used directly in the next step of the reaction. 1H NMR (CDCl3, 250MHz) δppm: 6.96 (d, J=8.5Hz, 2H), 6.63 (d, J=8.2Hz, 2H), 3.66 (s, 3H), 2.55 (t, J=7.6Hz, 2H), 2.31 (t, J=7.7Hz, 2H), 1.90 (q, J=7.6Hz, 2H).

67-56-1
782 suppliers
$7.29/5ml-f
15118-60-2
144 suppliers
$16.00/250mg

20637-09-6
88 suppliers
$18.00/100mg
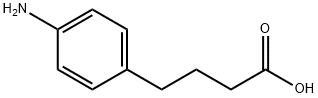
Yield: 96%
Reaction Conditions:
with sulfuric acid for 1.5 h;Reflux;
Steps:
130.1
A 250-mL round bottom flask was charged with 4-(4-aminophenyl)butyric acid (2.00 g, 11.2 mmol) in methanol (50 mL) and treated with cone, sulfuric acid (1 mL). The resultant mixture was heated to reflux for 1.5 h. After this time, methanol (-25 mL) was distilled off. The reaction was cooled to 60 °C and methyl tert-butyl ether was added. The mixture was allowed to slowly cool to room temperature, then diluted with hexanes (50 mL) to afford a white solid. The solid was dissolved in THF (6 mL)/water (4 mL) and treated with cone. NH4OH (6 mL). The mixture was diluted with dichloromethane (50 mL) and the layers separated. The organic layer was washed with saturated sodium chloride (5 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure to afford the title compound (2.07 g, 96%) as a brown solid. MW = 193.24. ]H NMR (DMSO-i, 500 MHz) δ 6.83-6.79 (m, 2H), 6.50-6.46 (m, 2H), 4.81 (s, 2H), 3.57 (s, 3H), 2.39 (t, J = 7.5 Hz, 2H), 2.25 (t, 7 = 7.5 Hz, 2H), 1.73 (quin, / = 7.5 Hz, 2H).
References:
Location in patent:Paragraph 0722

20637-02-9
7 suppliers
inquiry

20637-09-6
88 suppliers
$18.00/100mg

15118-60-2
144 suppliers
$16.00/250mg

20637-09-6
88 suppliers
$18.00/100mg

67-56-1
782 suppliers
$7.29/5ml-f
67857-97-0
7 suppliers
$57.00/25mg

5600-62-4
127 suppliers
$75.00/500mg

20637-09-6
88 suppliers
$18.00/100mg
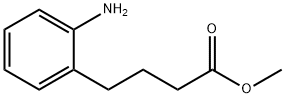
46320-17-6
1 suppliers
inquiry

15118-60-2
144 suppliers
$16.00/250mg
18107-18-1
210 suppliers
$33.00/5g

20637-09-6
88 suppliers
$18.00/100mg