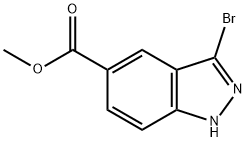
Methyl 3-bromoindazole-5-carboxylate synthesis
- Product Name:Methyl 3-bromoindazole-5-carboxylate
- CAS Number:1086391-06-1
- Molecular formula:C9H7BrN2O2
- Molecular Weight:255.07

473416-12-5

1086391-06-1
General procedure for the synthesis of methyl 3-bromo-1H-indazole-5-carboxylate from methyl 1H-indazole-5-carboxylate: a mixture of methyl 1H-indazole-5-carboxylate (1.7 g, 0.01 mol) with N-bromosuccinimide (NBS, 2.1 g, 0.012 mol) in tetrahydrofuran (THF, 10 ml) was stirred at room temperature overnight. After completion of the reaction, the mixture was concentrated to give a residue. To the residue was added dichloromethane (DCM, 5 ml), stirred for 30 min and filtered to give methyl 3-bromo-1H-indazole-5-carboxylate (1.9 g, 80% yield) as a light yellow solid.

473416-12-5
190 suppliers
$5.00/250mg

1086391-06-1
67 suppliers
$15.00/250mg
Yield:1086391-06-1 99%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione in diethyl ether at 20; for 0.5 h;
References:
Wang, Lei;Zhai, Lele;Chen, Jinyan;Gong, Yulin;Wang, Peng;Li, Huilin;She, Xuegong [Journal of Organic Chemistry,2022,vol. 87,# 5,p. 3177 - 3183] Location in patent:supporting information

61700-61-6
218 suppliers
$7.00/250mg

1086391-06-1
67 suppliers
$15.00/250mg