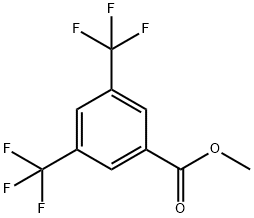
METHYL 3,5-BIS(TRIFLUOROMETHYL)BENZOATE synthesis
- Product Name:METHYL 3,5-BIS(TRIFLUOROMETHYL)BENZOATE
- CAS Number:26107-80-2
- Molecular formula:C10H6F6O2
- Molecular Weight:272.14

67-56-1
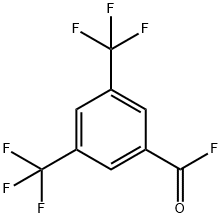
401-96-7

26107-80-2
To the reaction system was added 25.9 g (0.8 mol) of methanol, and after chlorine-fluorine exchange using HF according to the method of Example 2, 13.8 g (0.053 mol) of 3,5-bis(trifluoromethyl)benzoyl fluoride fraction was obtained, which was added slowly and dropwise to the reaction system over 60 minutes. Subsequently, the reaction mixture was stirred at 40°C for 4 hours. The generation of 14.2 g (0.052 mol) of methyl 3,5-bis(trifluoromethyl)benzoate was confirmed by gas chromatographic analysis (yield: 98%, selectivity: 100%). By vacuum distillation, 13.9 g (0.05 mol) of methyl 3,5-bis(trifluoromethyl)benzoate was isolated (yield: 96%). Examples 17-31 Various (fluoroalkyl)benzene derivatives were prepared with reference to the methods of Examples 2-16. To improve the purity of the product, Example 17 was purified by crystallization twice, and the final distillation step was repeated in the remaining Examples. The purity, residual halogen content and residual metal content of all (fluoroalkyl)benzene derivatives are summarized in Table 2.
Yield: 96%
Reaction Conditions:
at 40; for 5 h;
Steps:
4; 19 EXAMPLE 4; Production of methyl 3,5-bis(trifluoromethyl)benzoate
Into 25.9 g (0.8 mol) of methanol, 13.8 g (0.053 mol) of the fraction of 3,5-bis(trifluoromethyl)benzoyl fluoride obtained after the chlorine-fluorine exchange using HF in the same manner as in Example 2 was added dropwise over 60 min. Then, the reaction was allowed to proceed at 40°C for 4 h. By gas chromatographic analysis, the production of 14.2 g (0.052 mol) of the aimed methyl 3,5-bis(trifluoromethyl)benzoate was confirmed (yield: 98%, selectivity: 100%). By vacuum distillation, 13.9 g (0.05 mol) of methyl 3,5-bis(trifluoromethyl)benzoate was isolated (yield: 96%). EXAMPLES 17 to 31 In the same manner as in Examples 2 to 16, each (fluoroalkyl)benzene derivative was produced. To reduce the impurities, the crystallization was conducted twice in Example 17 and the final distillation was repeated in the other examples. The purity, residual halogen content and residual metal content of the (fluoroalkyl)benzene derivatives are collectively shown in Table 2
References:
MITSUBISHI GAS CHEMICAL COMPANY, INC. EP1500641, 2005, A1 Location in patent:Page 10; 15

67-56-1
782 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
328-70-1
440 suppliers
$10.00/25 g

26107-80-2
100 suppliers
$5.00/1g
