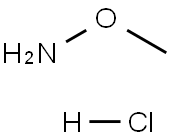
Methoxyamine Hydrochloride synthesis
- Product Name:Methoxyamine Hydrochloride
- CAS Number:593-56-6
- Molecular formula:CH5NO.ClH
- Molecular Weight:83.52
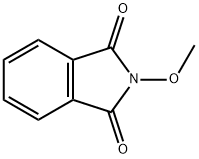
1914-20-1

593-56-6
General procedure: 500 mg of hydroxyphthalimide (Aldrich) was dissolved in 5 mL of dimethylformamide (DMF) under argon protection, followed by the addition of 0.2 mL of methylene iodide (Aldrich) and 0.5 mL of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, Aldrich) sequentially, slowly dropwise. The reaction mixture was stirred at 60 °C for 2 h and then cooled to room temperature and the reaction was quenched by addition of 2 N hydrochloric acid solution. The reaction solution was diluted with 20 mL of ethyl acetate, dried over magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography using ethyl acetate/hexane (1:5, v/v) as eluent and dried to give 407 mg of the target compound (yield: 75%). The compound was dissolved in 5 mL of dichloromethane and 0.11 mL of methylhydrazine (TCI) was slowly added at 0 °C. The reaction solution was stirred at room temperature for 2 hours and then cooled to 0°C again. The resulting solid was collected by filtration and 1 mL of 4 M dioxane hydrochloride solution (Aldrich) was added to the filtrate, filtered and dried to give 173 mg of solid product (yield: 90%). Under argon protection, 10 mg of the above solid was dissolved with 54 mg of SAC-0906 in 1 mL of pyridine (Aldrich) and stirred at 80 °C for 4 hours. After the reaction solution was cooled to room temperature, it was acidified by adding 2 N hydrochloric acid solution, extracted with 20 mL of ether, dried over magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography using ethyl acetate/hexane (1:5, v/v) as eluent to give the final methoxyamine hydrochloride SAC-1012 (48 mg, yield: 85%).1H-NMR (300 MHz, CDCl3) δ 5.91-5.76 (m, 2H), 5.34-5.32 (m, 1H) , 5.28-5.24 (m, 1H), 5.15 (m, 1H), 4.24-4.07 (m, 3H), 3.80 (s, 3H), 3.58-3.48 (m, 1H), 2.42-0.60 (m, 35H).
546-88-3
483 suppliers
$5.00/100mg
77-78-1
326 suppliers
$22.00/25g

593-56-6
635 suppliers
$8.00/100dtn(0.1g)
Yield:593-56-6 96.3%
Reaction Conditions:
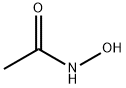
Stage #1: acetylhydroxamic acid;dimethyl sulfatewith sodium hydrogencarbonate;sodium hydroxide in water at 20; pH=7.5 - 8; for 3.5 h;
Stage #2: with hydrogenchloride in water;isopropyl alcohol at 20; pH=< 2; for 0.5 h;Temperature;pH-value;
Steps:
1-3 The preparation method of methoxyamine hydrochloride of this embodiment includes the following steps:
1) Add 0.2 mol sodium bicarbonate and 2.3 mol sodium hydroxide to 200 g of water and mix uniformly to obtain a composite alkali aqueous solution (ie, mixed aqueous solution);2) Add 108g (1.44mol) of acetohydroxamic acid and 252g of water to the reaction flask and mix to obtain a reaction solution. Control the temperature of the reaction solution to 20°C, add 163.4g (1.30mol) dimethyl sulfate dropwise, and add the compound dropwise at the same time. Alkaline aqueous solution, control the pH of the reaction system = 7.5-8.0, after 2 hours of dripping, the dimethyl sulfate dripping is completed and stirred for 0.5h. Then continue to add 18.1g (0.14mol) dimethyl sulfate dropwise, and at the same time add the composite alkali aqueous solution, control the pH of the reaction system = 7.5-8.0, stir for 0.5h after dropping, and then add 27.2g (0.22mol) dimethyl sulfate dropwise At the same time, the composite alkali aqueous solution was added dropwise, the pH of the reaction system was controlled to be 7.5-8.0, the dropping was completed and the reaction was stirred for 0.5 h, TLC monitored the completion of the reaction, and the reaction product liquid was obtained.3) The above reaction product liquid was heated to 60°C, kept for 1 hour (quenching dimethyl sulfate), and dropped to room temperature. 156.8g 90% concentrated sulfuric acid (containing 1.44mol H2SO4) was added dropwise, and reacted at 73°C. After 3 hours, the acetic acid was distilled off under reduced pressure at 60°C, and 500g ethanol was added to disperse the remainder. After that, alkali was added to adjust pH=10, stirred for 0.5h, filtered, and the filtrate was distilled at atmospheric pressure to collect 80-95°C. Then add 115g of 30% mass fraction of concentrated hydrochloric acid to adjust pH<2, evaporate to dryness, then add 60g of isopropanol, stir at room temperature for half an hour, filter, and dry the obtained solid at 50 to obtain 116.4g of finished product. The rate is 96.3%, and the gas phase detection purity is 99.5%.
References:
CN112851543,2021,A Location in patent:Paragraph 0038-0052

1914-20-1
12 suppliers
inquiry

593-56-6
635 suppliers
$8.00/100dtn(0.1g)
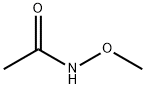
5806-90-6
25 suppliers
inquiry

593-56-6
635 suppliers
$8.00/100dtn(0.1g)
![Benzaldehyde, 4-nitro-, O-methyloxime, [C(E)]-](/CAS/20240320/GIF/54615-10-0.gif)
54615-10-0
0 suppliers
inquiry
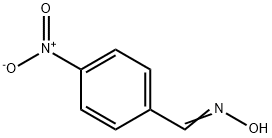
1129-37-9
98 suppliers
$18.00/1g

593-56-6
635 suppliers
$8.00/100dtn(0.1g)
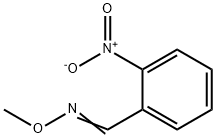
135436-97-4
1 suppliers
inquiry
![syn-2-Nitrobenzaldoxime [Deprotecting Agent]](/CAS/20180601/GIF/4836-00-4.gif)
4836-00-4
14 suppliers
$29.00/5g

593-56-6
635 suppliers
$8.00/100dtn(0.1g)