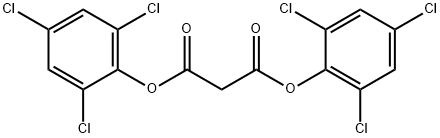
MALONIC ACID BIS(2,4,6-TRICHLOROPHENYL) ESTER synthesis
- Product Name:MALONIC ACID BIS(2,4,6-TRICHLOROPHENYL) ESTER
- CAS Number:15781-70-1
- Molecular formula:C15H6Cl6O4
- Molecular Weight:462.92
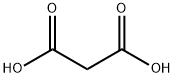
141-82-2
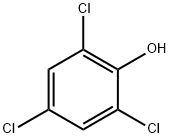
88-06-2

15781-70-1
The general procedure for the synthesis of bis(2,4,6-trichlorophenyl) malonate from malonic acid and 2,4,6-trichlorophenol was as follows: 2,4,6-trichlorophenol (15.8 g, 80 mmol) and malonic acid (4.1 g, 40 mmol) were added to phosphorus triclosamide (100 mL, 1.05 mol) according to the literature method. The reaction mixture was stirred under reflux conditions for 4 hours until the cessation of the escape of hydrogen chloride gas was confirmed by litmus test. The warm crystalline suspension was slowly poured into ice water (500 g) and stirred continuously for 30 minutes. Off-white crystals were collected by filtration and subsequently transferred to a mixture containing water (100 mL) and saturated aqueous sodium bicarbonate (50 mL). After stirring for 30 min, the crystals were filtered again, washed thoroughly with distilled water (3 x 40 mL) and finally dried under vacuum to afford the target product bis(2,4,6-trichlorophenyl) malonate (18.5 g, 95% yield) as a white fine solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.39 (4H, s, Ar-H), 4.05 (2H, s, CH2).

141-82-2
783 suppliers
$5.00/25g

88-06-2
285 suppliers
$10.00/10g

15781-70-1
118 suppliers
$5.00/100mg
Yield:15781-70-1 95%
Reaction Conditions:
with trichlorophosphate for 12 h;Heating / reflux;
Steps:
41; 45
A mixture of malonic acid (20 g, 192 mmol), 2,4,6-trichlorophenol (72 g, 365 mmol), and phosphorus oxychloride (38 mL, 403.2 mmol) was stirred at reflux for 12 hours. The reaction mixture was cooled to 7O0C and poured into ice water. The solid was collected by filtration, washed with water, and air dried to give malonic acid bis-(2,4,6-trichloro-phenyl) ester (85 g, 95%). A solution of malonic acid bis-(2,4,6-trichloro-phenyl) ester (85 g, 183.6 mmol) and ethyl 3- aminocrotonate (26.1 g, 202 mmol) in bromobenzene (100 mL) was stirred at reflux for 50 min. The reaction mixture was cooled to 5O0C and diluted with EtOAc (260 mL). The solid was collected by filtration, washed with water, and air dried to give 4,6-dihydroxy-2-methyl nicotinic acid ethyl ester (31 g, 86%).; A mixture of malonic acid (20 g, 192 mmol), 2,4,6-trichlorophenol (72 g, 365 mmol), and phosphorus oxychloride (38 ml_, 403.2 mmol) was stirred at reflux for 12 hours. The reaction mixture was cooled to 7O0C and poured into icy water. The solid was collected by filtration, washed with water, and air dried to give malonic acid bis-(2,4,6-trichloro-phenyl) ester (85 g, 95%). A solution of malonic acid bis-(2,4,6-trichloro-phenyl) ester (85 g, 184 mmol) and ethyl 3- aminocrotonate (26.08 g, 202 mmol) in bromobenzene (100 ml_) was stirred at reflux for 50 min. The reaction mixture was cooled to 5O0C and diluted with EtOAc (260 ml_). The solid was collected by filtration, washed with water, and air dried to give 4,6-dihydroxy-2-methyl nicotinic acid ethyl ester (31 g, 86%). A solution of 4,6-dihydroxy-2-methyl nicotinic acid ethyl ester (31.0 g, 157 mmol) in phosphorus oxychloride (60.0 mL, 629 mmol) was stirred at reflux for 1.5 hours. The extra phosphorus oxychloride was removed using a rotary evaporator and the reaction mixture was poured into icy water. The solid was removed by filtration. The filtrate was extracted with dichloromethane (3x100 mL), and concentrated using a rotary evaporator. The residue was further purified by column (SiO2, Hexanes/EtOAc = 5:1 ) to yield 4,6-dichloro-2-methyl nicotinic acid ethyl ester (16.9 g, 46%). A solution of 4,6-dichloro-2-methyl nicotinic acid ethyl ester (16.9 g, 71.3 mmol) in MeOH (60 mL) was mixed with sodium methoxide (58 mL, 257 mmol) and stirred at reflux for 12 hours. The reaction was quenched by adding AcOH (50 mL), diluted with water (200 mL), extracted with dichloromethane (3x100 mL), and concentrated using a rotary evaporator. The residue was further purified by EPO
References:
RESVERLOGIX CORP.;JOHANSSON, Jan, O.;HANSEN, Henrik, C.;CHIACCHIA, Fabrizio, S.;WONG, Norman, C.W. WO2007/16525, 2007, A2 Location in patent:Page/Page column 94; 101-103