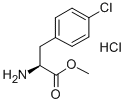
L-4-CHLOROPHENYLALANINE METHYL ESTER HCL synthesis
- Product Name:L-4-CHLOROPHENYLALANINE METHYL ESTER HCL
- CAS Number:63024-26-0
- Molecular formula:C10H13Cl2NO2
- Molecular Weight:250.12
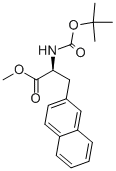
176896-73-4

63024-26-0
Step B: Methyl (2S)-2-(tert-butoxycarbonylamino)-3-(naphthalen-2-yl)propionate (5.52 g, 15.85 mmol) and 20 mL of ethyl acetate were added to a 500 mL round-bottom flask fitted with a magnetic stirrer. 80 mL of 2.8 M hydrogen chloride in ethyl acetate solution was slowly added under stirring and the reaction was carried out under nitrogen protection for 2.5 hours. Upon completion of the reaction, the reaction mixture was concentrated to dryness under reduced pressure to give the crude product. The crude product was dissolved in an appropriate amount of ethyl acetate, stirred for 10 minutes and filtered to collect the solid. The resulting solid was dried under vacuum at 40 °C to give (2S)-2-amino-3-(naphthalen-2-yl)propanoic acid methyl ester hydrochloride 3.91 g in 93% yield as a white solid.HPLC-MS analysis: retention time (Rt) = 3.70 min, molecular ion peak (M + 1) = 230, % ELS area = 100%.

176896-73-4
9 suppliers
inquiry

63024-26-0
40 suppliers
$36.21/1G
Yield:63024-26-0 93%
Reaction Conditions:
with hydrogenchloride in ethyl acetate; for 2.5 h;
Steps:
31.B
Step B: (2S)-2-tert-Butoxycarbonylamino-3-(2-naphthyl)propionic acid methyl ester (5.52 g, theoretically 15.85 mmol) is dissolved in 20 ml of ethyl acetate in a 500 ml flask equipped with a magnetic stirrer. To the stirred solution is added 80 ml of 2.8 M hydrogen chloride in ethyl acetate and the reaction is stirred for 2.5 hours under nitrogen. The clear mixture is concentrated in vacuo to give a solid, which is taken up in ethyl acetate, stirred for 10 min and filtered. The solid is dried in vacuo at 40° C. to give 3.91 g (93%) of (2S)-2-Amino-3-(2-naphthyl)propionic acid methyl ester hydrochloride as a white solid. HPLC-MS: Rt=3.70 min., (M+1)=230, % Area by ELS=100
References:
US2007/185128,2007,A1 Location in patent:Page/Page column 27
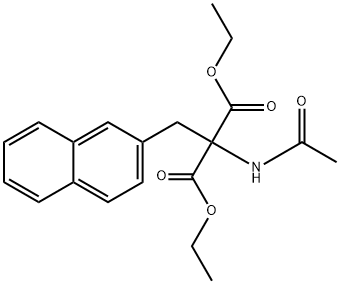
37447-33-9
3 suppliers
inquiry

63024-26-0
40 suppliers
$36.21/1G
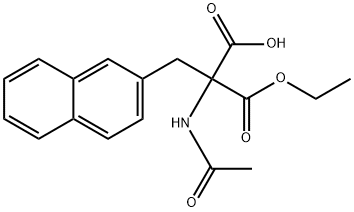
136015-50-4
0 suppliers
inquiry

63024-26-0
40 suppliers
$36.21/1G
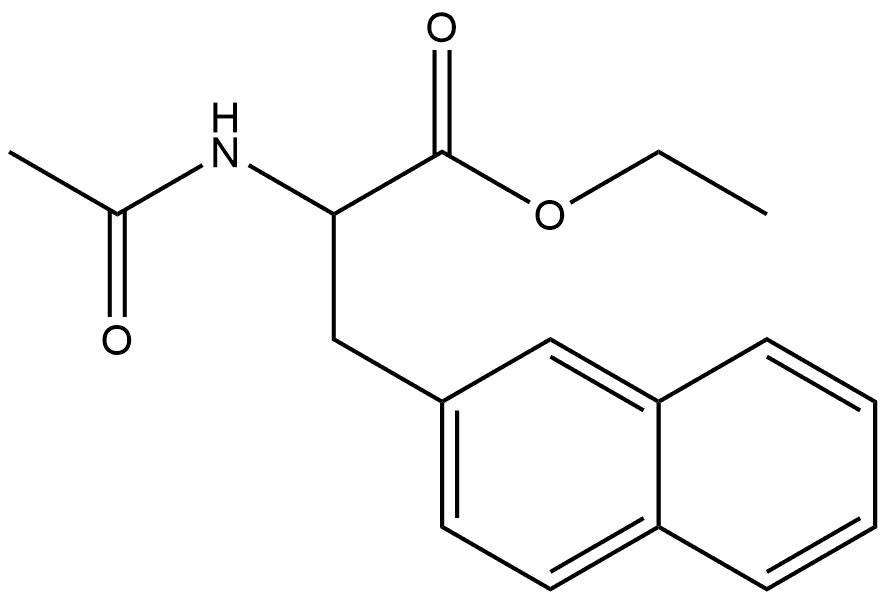
172214-89-0
0 suppliers
inquiry

63024-26-0
40 suppliers
$36.21/1G