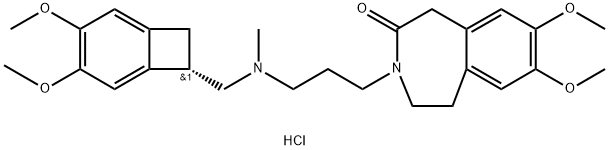
Ivabradine hydrochloride synthesis
- Product Name:Ivabradine hydrochloride
- CAS Number:148849-67-6
- Molecular formula:C27H37ClN2O5
- Molecular Weight:505.05
![3-[3-[[[(7S)-3,4-DiMethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]Methyl]MethylaMino]propyl]-1,3-dihydro-7,8-diMethoxy-H-3-benzazepin-2-one](/CAS/GIF/1086026-31-4.gif)
1086026-31-4

148849-67-6
1. 567 g of (S)-3-(3-(((3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl)(methyl)amino)propyl)-7,8-dimethoxy-1H-benzo[d]azepin-2(3H)-one was added to a reaction flask. 2. 2.55 L of glacial acetic acid and 141 g of palladium-carbon was added with thorough stirring and cooled to 20±5°C. 3. After evacuation, replace the nitrogen gas 3 times. 4. Control the temperature at 15~25°C and react under atmospheric pressure of hydrogen for 23 h. 5. Filter the reaction mixture through diatomaceous earth, and rinse the filter cake with glacial acetic acid. 6. Mix the filtrate with purified water and ethyl acetate, and mix thoroughly. 7. Adjust the pH to 9-10 with sodium hydroxide solution. 8. Separate the liquids, and the aqueous phase was extracted with ethyl acetate, and the organic phase was combined. 9. The organic phase was washed with saturated sodium bicarbonate solution and saturated sodium chloride solution in turn. 10. The organic phase was dried overnight, filtered, and the filter cake was rinsed with ethyl acetate. 11. The filtrate was dried by rotary evaporation to give 403.9 g of ivabradine, with a yield of 70.9% and a purity of 96.3% by HPLC. 12. To a 10L reaction flask, 402 g of ivabradine and 6L of ethyl acetate were added and stirred. 13. 6L of ethyl acetate was added to a 10L reaction flask, stirred until complete dissolution, and cooled to 0~10°C. 13. 172.2g of 20wt% hydrogen chloride isopropanol solution was added, and a large amount of white solid precipitate was observed. 14. After stirring for 1 hour, it was filtered, and the filter cake was rinsed with ethyl acetate. 15. The solid was dried in vacuum for 15 hours, and 405.1g of white solid Ivabradine HCL was obtained, with the yields of 93.5% and HPLC purity of 99.3%. 16. HPLC purity was 99.8%.
![3-[3-[[[(7S)-3,4-DiMethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]Methyl]MethylaMino]propyl]-1,3-dihydro-7,8-diMethoxy-H-3-benzazepin-2-one](/CAS/GIF/1086026-31-4.gif)
1086026-31-4
74 suppliers
$200.00/5mg

148849-67-6
453 suppliers
$5.00/10mg
Yield:148849-67-6 93.5%
Reaction Conditions:
Stage #1: 3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-onewith palladium 10% on activated carbon;hydrogen;acetic acid at 15 - 25; for 23 h;
Stage #2: with hydrogenchloride in ethyl acetate;isopropyl alcohol at 0 - 10; for 1 h;
Steps:
3; 4 Steps:
567 g of the dehydrogenated ivabradine obtained in Example 1 was added to the reaction flask.2.55L glacial acetic acid,141g palladium on carbon,Stir well and cool to 20±5°C.After vacuuming, the nitrogen was replaced 3 times.Hydrogen replacement 3 times; control temperature 15~25 ° C, atmospheric hydrogen After 23 hours of reaction in the environment.The mixture was filtered through celite, and the filter cake was rinsed with glacial acetic acid.Purified water and ethyl acetate were added to the oil, stirred well, and the pH was adjusted to 9 to 10 with a sodium hydroxide solution. Liquid separation, water phaseExtracted with ethyl acetate, combined with organic phase, washed with saturated sodium bicarbonate, washed with saturated sodium chlorideAfter stirring and drying overnight, suction filtration, the filter cake was rinsed with ethyl acetate, and the filtrate was spun dry to obtain 403.9 g of ivabradine, the yield was 70.9%.HPLC purity: 96.3%. Into a 10 L reaction flask was placed 402 g of ivabradine, 6 L of ethyl acetate,Stir until fully dissolved,Cool down to 0~10 °C,Add 172.2gHydrogen chloride isopropanol solution (20wt%),A large amount of white solid is precipitated,Stir for 1 hour,Filtered, the filter cake was rinsed with ethyl acetate.The material was vacuum dried for 15 hours to obtain a white solid pulverized ivabradine 405.1 g,Yield 93.5%, HPLC purity: 99.8%.
References:
CN108424390,2018,A Location in patent:Paragraph 0057; 0059-0062; 0063-0069
![3-[3-({[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}(methyl)amino)propyl]-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one hydrochloride](/CAS/20200331/GIF/1086026-38-1.gif)
1086026-38-1
10 suppliers
inquiry

148849-67-6
453 suppliers
$5.00/10mg
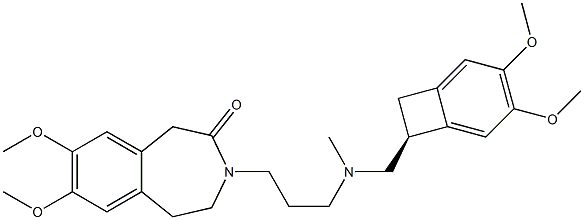
155974-00-8
101 suppliers
inquiry

148849-67-6
453 suppliers
$5.00/10mg
![7,8-Dimethoxy-1,3,4,5-tetrahydrobenzo[d]azepin-2-one](/CAS/GIF/20925-64-8.gif)
20925-64-8
98 suppliers
inquiry

1031767-71-1
4 suppliers
inquiry

148849-67-6
453 suppliers
$5.00/10mg
![(1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride](/CAS/GIF/866783-13-3.gif)
866783-13-3
277 suppliers
$50.00/100mg
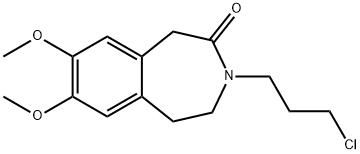
85175-65-1
72 suppliers
inquiry

148849-67-6
453 suppliers
$5.00/10mg