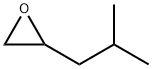
isobutyloxirane synthesis
- Product Name:isobutyloxirane
- CAS Number:23850-78-4
- Molecular formula:C6H12O
- Molecular Weight:100.16
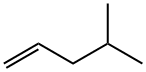
691-37-2
66 suppliers
$30.39/10g

23850-78-4
8 suppliers
inquiry
Yield: 96%
Reaction Conditions:
with dihydrogen peroxide;cobalt(II) acetate in water at 20; for 2 h;Ionic liquid;Green chemistry;
Steps:
General procedure for epoxidation reactions
General procedure: For a typical experiment, alkene (10 mmol), Co(OAc)2(1 mmol), and an aqueous solution of H2O2 (30 %,11 mmol) were sequentially added to 10 mL of [C12py][PF6]. The obtained mixture was stirred vigorously at room temperature for the appropriate time (Table 2), the reaction progress was monitored by GC. Upon completion ofthe reaction, cyclohexane was used to extract the organic compounds (3 × 10 mL). The cyclohexane solution was washed with water (3 × 10 mL), and then dried over anhydrous Na2SO4. The solvent was removed and the residue was distilled under vacuum to give the desired pure product.The rest of the ionic liquid and the catalyst were recovered by decantation of water produced in the reaction and concentration under vacuum. Fresh substrates were then recharged to the recovered catalytic system and then recycled under identical reaction conditions. The target substrates were characterized by NMR and Elemental analysisor compared with their authentic samples. Spectroscopic data for selected products are as follows.
References:
Hu, Yu-Lin;Liu, Yi-Wen;Li, De-Jiang [Journal of the Iranian Chemical Society,2015,vol. 12,# 12,p. 2179 - 2184]
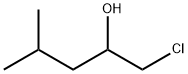
84055-72-1
0 suppliers
inquiry

23850-78-4
8 suppliers
inquiry