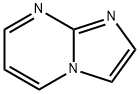
Imidazo[1,2-a]pyrimidine synthesis
- Product Name:Imidazo[1,2-a]pyrimidine
- CAS Number:274-95-3
- Molecular formula:C6H5N3
- Molecular Weight:119.12
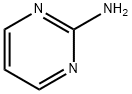
109-12-6
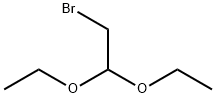
2032-35-1
![Imidazo[1,2-a]pyrimidine](/CAS/GIF/274-95-3.gif)
274-95-3
In a 250-mL three-necked round-bottomed flask, 2-aminopyrimidine (5 g, 0.052 mol), bromoacetaldehyde diethyl acetal (20.7 g, 0.104 mol), 48% aqueous hydrobromic acid (5 mL), and ethanol (50 mL) were added sequentially. The reaction mixture was stirred at room temperature for 18 hours. After completion of the reaction, the reaction solution was cooled to room temperature. Subsequently, the reaction mixture was adsorbed onto silica gel and purified by column chromatography (eluent: dichloromethane/methanol), resulting in 5 g of imidazo[1,2-a]pyrimidine in about XX% yield.

109-12-6
447 suppliers
$6.00/10g

2032-35-1
485 suppliers
$5.00/10g
![Imidazo[1,2-a]pyrimidine](/CAS/GIF/274-95-3.gif)
274-95-3
170 suppliers
$11.00/250mg
Yield:274-95-3 5 g
Reaction Conditions:
with hydrogen bromide in ethanol;water; for 18 h;Reflux;
Steps:
1.1.1 Synthesis of intermediate C-1
In a 250 ml three-neck round bottom flask, 2-aminopyrimidine (5 g),Bromoacetaldehyde diethyl acetal (20.7 g), 48% aqueous solution of bromic acid (5 ml)Ethanol (50 ml) was added and the mixture was refluxed with stirring for 18 hours. The reaction solution was cooled to room temperature Silica gel was adsorbed. Through column separation using dichloromethane and methanol5 g of the title compound was obtained.
References:
KR2017/126059,2017,A Location in patent:Paragraph 0069; 0072; 0073; 0076; 0077

109-12-6
447 suppliers
$6.00/10g

107-20-0
0 suppliers
$22.60/5ml
![Imidazo[1,2-a]pyrimidine](/CAS/GIF/274-95-3.gif)
274-95-3
170 suppliers
$11.00/250mg