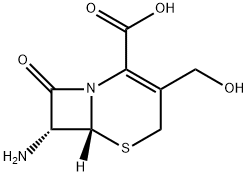
Hydroxymethyl-7-Aminocephalosporanic acid synthesis
- Product Name:Hydroxymethyl-7-Aminocephalosporanic acid
- CAS Number:15690-38-7
- Molecular formula:C8H10N2O4S
- Molecular Weight:230.24
957-68-6

15690-38-7
General procedure for the synthesis of (6R,7R)-7-amino-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid from 7-aminocephalosporanic acid (7-ACA): 27.2 g (0.1 mol) of 7-ACA were accurately weighed and placed in a reaction flask, to which 50 mL of methanol and 50 mL of water were added as solvents. 3.0 g of tetra-n-butyl ammonium chloride was added while stirring at a temperature of -5 to 5 °C. Subsequently, 2 mol/L sodium hydroxide solution was added slowly dropwise. After the dropwise addition was completed, the reaction temperature was maintained and stirring was continued for 0.5 to 1 hour. Upon completion of the reaction, the pH of the reaction solution was adjusted to neutral with 30% hydrochloric acid, at which point a solid precipitated. The solid product was collected by diafiltration and washed with anhydrous ethanol. Eventually, 20.7 g of dry white solid product was obtained. The product was analyzed by high performance liquid chromatography (HPLC) and the purity of the product was 97.35% (area normalization method) and the content of lactone impurities was 0.33%.

957-68-6
457 suppliers
$14.00/5g

15690-38-7
153 suppliers
$9.00/250mg
Yield:15690-38-7 90.1%
Reaction Conditions:
with tetrabutyl-ammonium chloride;sodium hydroxide in methanol;water at -5 - 5;
Steps:
1 Synthesis of 7-Amino-3-Hydroxymethylcephalosporanic Acid (7-DACA, Compound 6)
Weigh 7-ACA 27.2g (0.1mol),Add 50mL of methanol and 50mL of water,Add 3.0g tetra-n-butane at -5-5°CAmmonium chloride stirring to dissolve,Maintain the addition of 2mol/L sodium hydroxide solution 105mL at this temperature.After dripping, maintain the temperature and continue stirring for 0.5-1 hour.Then add 30% hydrochloric acid to adjust the pH to neutral,Precipitation of solids, suction filtration, anhydrous ethanol washing,20.7g of dried white solidYield 90.1%,HPLC purity analysis 97.35% (area normalization method),Lactone impurity 0.33%.
References:
Shandong Luoxin Pharmaceutical Group Co., Ltd.;Wang Jinxing;Hou Xiaolong;Xu Qinyan CN105131017, 2017, B Location in patent:Paragraph 0041-0042
