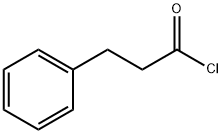
Hydrocinnamoyl chloride synthesis
- Product Name:Hydrocinnamoyl chloride
- CAS Number:645-45-4
- Molecular formula:C9H9ClO
- Molecular Weight:168.62
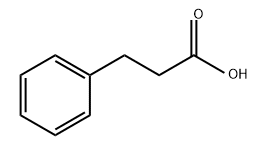
501-52-0

645-45-4
General procedure for the synthesis of hydrocinnamoyl chloride from hydrogenated cinnamic acid: To a 200 mL flask equipped with a stir bar and an argon inlet septum were added hydrogenated cinnamic acid (5.00 g, 33.3 mmol), N,N-dimethylformamide (DMF, 0.1 mL), and dichloromethane (CH2Cl2, 30 mL). Oxalyl chloride ((COCl)2, 2 M in CH2Cl2, 20.0 mL, 40.0 mmol) was slowly added to the resulting solution over 30 min. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the solvent was removed by vacuum concentration to afford 3-phenylpropionyl chloride (74, 5.9 g, yellow oil) in 100% yield. The purity of the product was confirmed to be 99% by high performance liquid chromatography (HPLC) analysis (mobile phase ratio: water:acetonitrile:methanol = 15:10:75) with a retention time of 5.3 min.

501-52-0
510 suppliers
$14.14/1gm:

645-45-4
354 suppliers
$15.00/5g
Yield:645-45-4 100%
Reaction Conditions:
with oxalyl dichloride in dichloromethane at 20;
Steps:
3-Phenylpropanoyl chloride (74).
3-Phenylpropanoyl chloride (74). A 200 mL flask fitted with a stir-bar and septum with an Ar inlet was charged with hydrocinnamic acid (5.00 g, 33.3 mmol), DMF (0.1 mL), and CH2CI2 (30 mL). To the resultant solution was added (COCI)2 (2M in CH2CI2, 20.0 mL, 40.0 mmol) over 30 min. The mixture stirred overnight at rt, and then was concentrated in vacuo to give 5.9 g of 74 as a yellow oil (100%). HPLC analysis (15:10:75 H2O:A1 :MeOH) showed a purity of 99% with a retention time of 5.3 min.
References:
WO2013/101911,2013,A2 Location in patent:Page/Page column 57
16537-10-3
5 suppliers
inquiry

645-45-4
354 suppliers
$15.00/5g
330-12-1
249 suppliers
$15.00/10g

645-45-4
354 suppliers
$15.00/5g
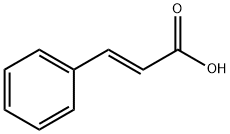
140-10-3
638 suppliers
$5.00/10g

645-45-4
354 suppliers
$15.00/5g
28048-94-4
3 suppliers
inquiry

645-45-4
354 suppliers
$15.00/5g