Hesperetin synthesis
- Product Name:Hesperetin
- CAS Number:520-33-2
- Molecular formula:C16H14O6
- Molecular Weight:302.28
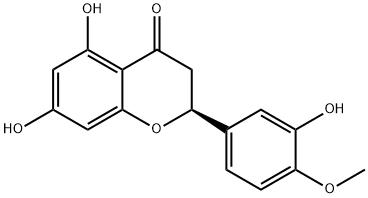
520-26-3

520-33-2
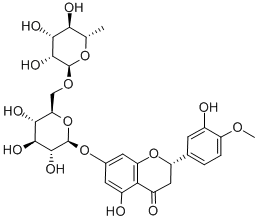
Generic method: compound 2 was synthesized by the method reported in the literature. The raw material (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy )benzodihydropyran-4-one (3.5 g, 5.7 mmol) was mixed with 10 mL of concentrated sulfuric acid in 280 mL of anhydrous methanol and the reaction was stirred at 60 °C for 7.5 hours. Upon completion of the reaction, 1.2 L of ethyl acetate was added to the reaction mixture and extracted at 20 °C. Subsequently, the organic phase was washed with 420 mL of water and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give a light yellow powdery crude product. The crude product was dissolved in 70 mL of acetone and then slowly added dropwise (over 60 minutes) to a pre-stirred 700 mL solution consisting of water and acetic acid mixed at 150:1 by volume, maintaining the temperature at 95°C. After dropwise addition, the mixed slurry was cooled to 45°C, the product was collected by filtration and dried under vacuum.

520-26-3
661 suppliers
$5.00/250mg

520-33-2
422 suppliers
$5.00/50mg
Yield:520-33-2 87%
Reaction Conditions:
with sulfuric acid in ethanol; for 8 h;Reflux;
Steps:
1 Synthesis of compound 1:
Weigh hesperidin (72g, about 0.12mol) into a 1000mL round-bottomed flask, add 720mL of 10% concentrated sulfuric acid ethanol solution, place in an oil bath and stir well, and heat to reflux. After 8 hours of reaction, after cooling, pour Into ice water, a solid precipitated, left to stand, suction filtered, and dried.The crude product was heated and dissolved with ethanol, and the activated carbon was decolorized and filtered.The filtrate was recovered under reduced pressure. The solid was recrystallized from ethanol and dichloromethane.A white solid was precipitated, suction filtered, and dried to obtain a hesperetin powder (31.5 g) in a yield of 87%.
References:
CN110372657,2019,A Location in patent:Paragraph 0036-0041
79566-13-5
8 suppliers
inquiry

520-26-3
661 suppliers
$5.00/250mg
![2H-1-Benzopyran-2-one, 7-[[6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-4-methyl-](/CAS/20210305/GIF/1356391-89-3.gif)
1356391-89-3
0 suppliers
inquiry

520-33-2
422 suppliers
$5.00/50mg
75679-30-0
8 suppliers
inquiry

520-33-2
422 suppliers
$5.00/50mg
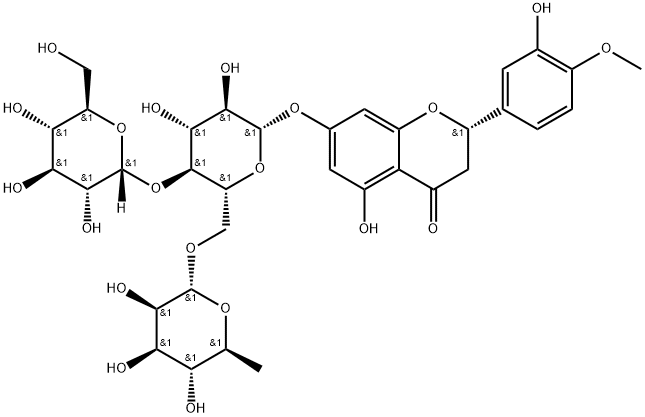
161713-86-6
47 suppliers
$95.76/5MG

520-26-3
661 suppliers
$5.00/250mg

520-33-2
422 suppliers
$5.00/50mg

71-23-8
793 suppliers
$12.00/100g

520-26-3
661 suppliers
$5.00/250mg
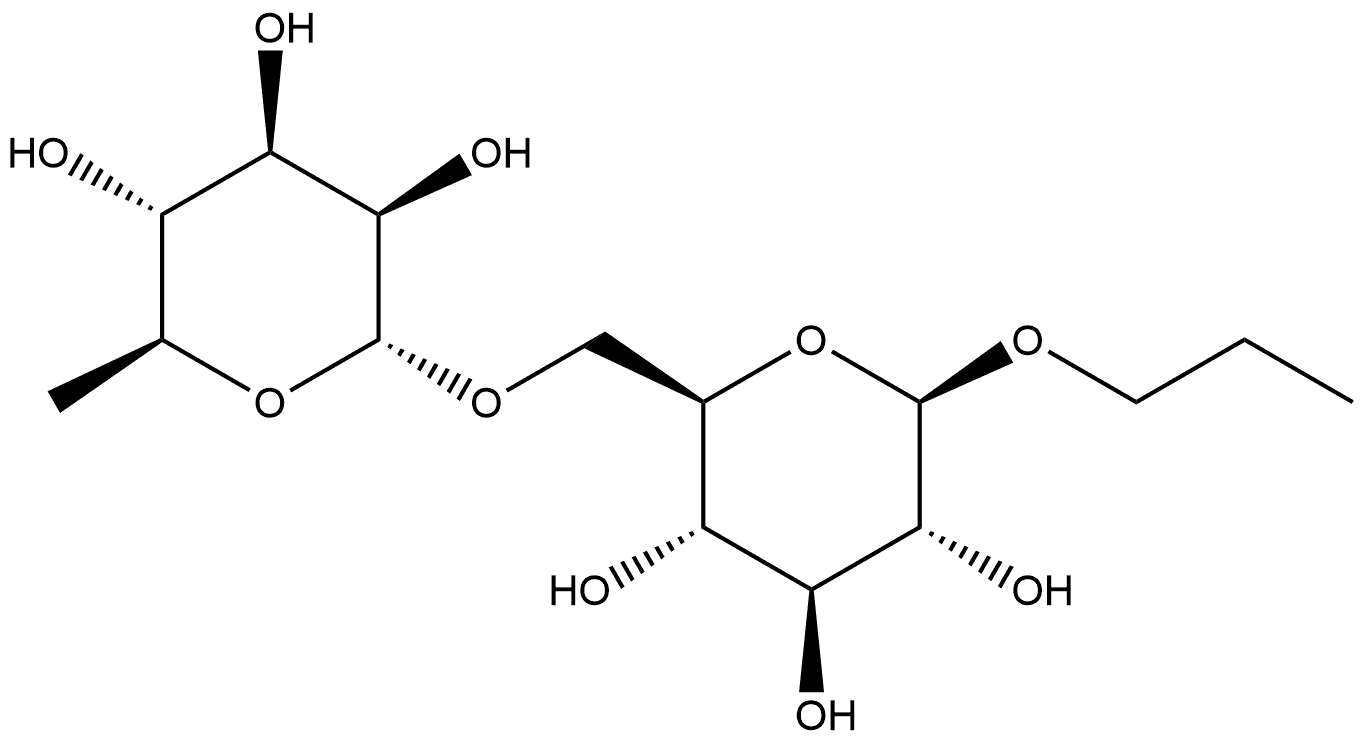
168172-05-2
0 suppliers
inquiry

520-33-2
422 suppliers
$5.00/50mg