
HA14-1 synthesis
- Product Name:HA14-1
- CAS Number:65673-63-4
- Molecular formula:C17H17BrN2O5
- Molecular Weight:409.23
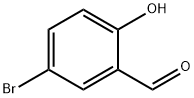
105-56-6
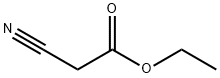
1761-61-1

65673-63-4
GENERAL METHODS: 1,1,3,3-Tetramethylguanidine (TMG, 3.5 mmol) was added to a mixture of 5-bromosalicylaldehyde (SA, 1 mmol), ethyl cyanoacetate (MN, 1 mmol), and 2,3-diaminopyridine (DAP, 1 mmol) under pure conditions. The reaction mixture was stirred at room temperature and the reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, distilled water (15 mL) was added to the reaction mixture and stirring was continued until a free-flowing solid was formed. The solid was collected by filtration and washed sequentially with water and hexane. The crude product was purified by recrystallization from ethanol solution: the crude product was dissolved in boiling ethanol to saturation and hot filtered to remove undissolved solid. The hot filtrate was cooled to room temperature to give an elementally analytically pure crystalline product, rel-(αR,4R)-2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-carbonyl)-4H-benzofuran-3-carboxylic acid. Similar experimental procedures were used to synthesize all 2-amino-4H-benzofuran derivatives.

105-56-6
457 suppliers
$10.00/5ml

1761-61-1
390 suppliers
$5.00/5g

65673-63-4
140 suppliers
$25.00/5mg
Yield:65673-63-4 94%
Reaction Conditions:
with N,N,N',N'-tetramethylguanidine in neat liquid at 20; for 0.583333 h;
Steps:
26 General procedure for synthesis of (2-amino-3-cyano-4H-chromene-4-yl)phosphonates (1-24) and 2-amino-4H-chromenes (25-32)
General procedure: TMG (3.5 mmol) was added to a mixture of SA (1 mmol), MN (1 mmol) and DAP (1 mmol) in neat condition. The resulting mixture was stirred at room temperature. After completion of the reaction (monitored by TLC), distilled water (15 mL) was added to the reaction mixture and stirring continued till a free flowing solid was obtained. It was filtered and then washed successively with water, n-hexane. The crude product was purified by recrystallization from ethanol solution. For this, the crude product was saturated in boiling ethanol and then the solution mixture was filtered when it was hot to remove the undissolved solid. The hot filtrate cooled down to room temperature to afford elemental analytically pure crystalline product. The similar experimental procedures were adopted for the synthesis of all the 2-amino-4H-chrormens.
References:
Kalla, Reddi Mohan Naidu;Byeon, Seong Jin;Heo, Min Seon;Kim, Il [Tetrahedron,2013,vol. 69,# 49,p. 10544 - 10551]
