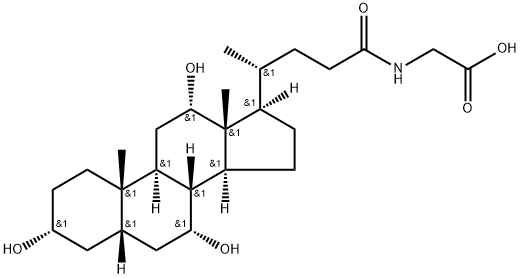
Glycocholic acid synthesis
- Product Name:Glycocholic acid
- CAS Number:475-31-0
- Molecular formula:C26H43NO6
- Molecular Weight:465.63
517904-33-5

475-31-0
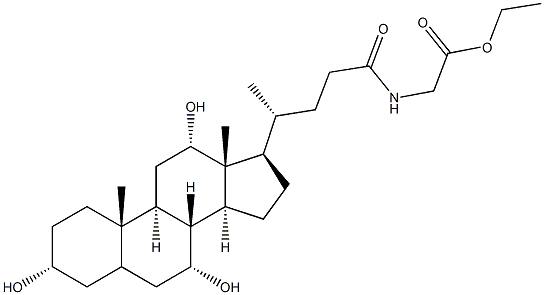
Example 3: General procedure for the synthesis of ((R)-4-((3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren[a]ethene-17-yl)pentanoyl)glycine from glycocholic acid ethyl ester (GCAEE): 1. 1.89 g of NaOH was dissolved in 189 ml of water at ambient temperature in a clean 1 liter dry glass flask and stirred until completely dissolved. 2. 16.9 g of purified wet GCAEE was added to the above solution and the suspension was stirred at 20 to 28°C for about 12 hours. 3. Adjust the pH of the clarified solution to between 7.0 and 7.5 using 50 ml of 1N HCl. 4. wash the aqueous solution twice with 75 ml of ethyl acetate at 35 to 40 °C. 5. Heat the separated aqueous solution under vacuum to remove the ethyl acetate. 6. the resulting aqueous solution was diluted with 100 ml of water, heated at 35 to 40 °C and the pH was adjusted to between 2.0 and 2.5 with 1N HCl to precipitate pure GCA. 7. The wet product was dried under vacuum at 50 to 60 °C to give 14 g of the sesquihydrate product in 92.3% dry yield. 8. TLC analysis showed that the purity of CA was less than 0.4%, GGCAEE was less than 0.1%, total other impurities were less than 0.2%, and no glycine was observed.

517904-33-5
31 suppliers
inquiry

475-31-0
331 suppliers
$19.00/100mg
Yield:475-31-0 92.3%
Reaction Conditions:
Stage #1: ethyl glycocholatewith lithium hydroxide monohydrate;sodium hydroxide at 20 - 28;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate; pH=7 - 7.5;
Steps:
3
Example 3Hydrolysis and precipitation of GCAAdded into a clean and dry 1 -litre glass flask are:- water 189 ml- NaOH 1.89 gStirring is carried out for about 1 hour in such a manner that all the sodium hydroxide dissolves, at ambient temperature.Once the solution is complete, 16.9 g of purified wet GCAEE are added and the suspension is stirred for about 12 hours, at a temperature comprised between 20 and 28°C.Then, the clear solution is taken to a pH comprised between 7.0 and 7.5 with about 50ml of HCl IN. The aqueous solution is washed twice using ethyl acetate75 ml, at a temperature between 35 and 40 °C.Thus, the separated aqueous solution is heated under vacuum to eliminate the ethyl acetate. The aqueous solution thus obtained is diluted with 100 ml of water, and the pure GCA is obtained by heating the solution between 35 and 40 °C and precipitation with HCl IN, up to a pH comprised between 2.0 and 2.5.The wet product is dried under vacuum between 50 and 60° C.The dry yield of the sesquihydrate product is 14 g, equivalent to 92.3% stoichiometric.TLC purityCA lower than 0.4 % - GGCAEE lower than 0.1 % - Other impurities lower than0.2 % in total. Glycine not observable.
References:
WO2010/128472,2010,A1 Location in patent:Page/Page column 6
81-25-4
555 suppliers
$5.00/100mg

623-33-6
657 suppliers
$5.00/10g

475-31-0
331 suppliers
$19.00/100mg

80948-49-8
0 suppliers
inquiry

475-31-0
331 suppliers
$19.00/100mg
![2,5-Pyrrolidinedione, 1-[[(3α,5β,7α,12α)-3,7,12-trihydroxy-24-oxocholan-24-yl]oxy]-](/CAS/20210305/GIF/70090-26-5.gif)
70090-26-5
2 suppliers
inquiry

475-31-0
331 suppliers
$19.00/100mg

81-25-4
555 suppliers
$5.00/100mg

475-31-0
331 suppliers
$19.00/100mg