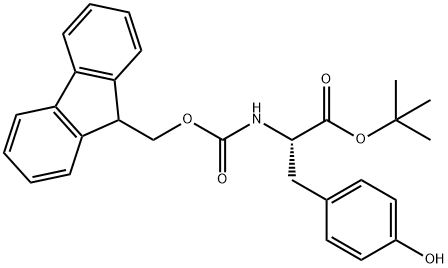
Fmoc-Tyr-OtBu synthesis
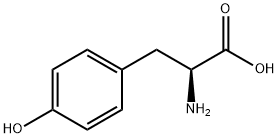
- Product Name:Fmoc-Tyr-OtBu
- CAS Number:133852-23-0
- Molecular formula:C28H29NO5
- Molecular Weight:459.53

16874-12-7
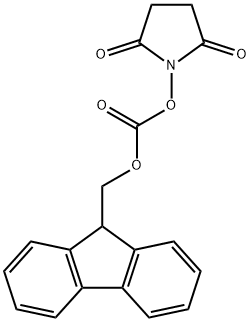
82911-69-1

133852-23-0
General procedure: tert-butyl L-tyrosine (1.00 g, 4.21 mmol) and sodium bicarbonate (354 mg, 4.21 mmol) were suspended in 1,4-dioxane/water (1:1, 20 mL) with stirring. To this suspension was added 9-fluorenylmethyl-N-succinimidyl carbonate (1.42 g, 4.21 mmol), and the resulting mixture was stirred at room temperature for 18 hours. Upon completion of the reaction, the solvent was concentrated under reduced pressure to about 10 mL. cold 1N hydrochloric acid (50 mL) was added and the product was extracted with ethyl acetate (3 x 50 mL). The organic phases were combined, washed sequentially with water and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to give the colorless solid product 83 (1.94 g, 100%), which could be used in the next reaction without further purification. The product was confirmed by 1H NMR (400 MHz, CDCl3): δ 1.43 (s, 9H), 2.97-3.06 (m, 2H), 4.21 (bt, J = 7.1 Hz, 1H), 4.33 (dd, J = 7.1, 10.5 Hz, 1H), 4.41-4.53 (m, 2H), 5.01 (bs, 1H), 5.30 (d, J = 8.2 Hz, 1H), 6.73 (d, J = 8.5 Hz, 2H), 7.00 (d, J = 8.5 Hz, 2H), 7.31 (t, J = 7.5 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.57 (dd, J = 3.3, 7.3 Hz, 2H), 7.76 (d, J = 7.5 Hz 2H).

16874-12-7
251 suppliers
$14.00/1G

82911-69-1
648 suppliers
$7.00/25g

133852-23-0
62 suppliers
inquiry
Yield: 100%
Reaction Conditions:
Stage #1:Tyr(tBu);N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide with sodium hydrogencarbonate in 1,4-dioxane;water at 20; for 18 h;
Stage #2: with hydrogenchloride;water in 1,4-dioxane
Steps:
16
[00284] tButyl N-(9-fluorenylmethoxycarbonyl)-L-tyrosine (83): To a stirring suspension of L-tyrosine O-f-butyl ester (LOOg, 4.21 mmol) and NaHCO3 (354 mg, 4.21 mmol) in 1 ,4-dioxane/water (1 :1 , 20 ml_) was added 9-fluorenylmethyl-N-succinimidyl carbonate (1.42 g, 4.21 mmol) and the resulting mixture was stirred for 18 hr at room temperature. The solvent was reduced to 10 ml. followed by the addition of 50 ml. of cold 1 N HCl. The product was extracted with ethyl acetate (3x). The organic extracts were washed with H2O and saturated aqueous NaCl then dried over Na2SO4. The solution was then concentrated after filtration to give the colourless solid 83 (1.94 g, 100%) which was used without purification: 1H NMR (400 MHz, CDCI3) δ 1.43 (s, 9H), 2.97-3.06 (m, 2H), 4.21 (bt, J=7.1 , 1 H), 4.33 (dd, J=7.1 , 10.5, 1 H), 4.41-4.53 (m, 2H), 5.01 (bs, 1 H), 5.30, (d, J=8.2, 1 H), 6.73 (d, J=8.5, 2H), 7.00 (d, J=8.5, 2H), 7.31 (t, J=7.5, 2H), 7.40 (t, J=7.5, 2H), 7.57 (dd, J=3.3, 7.3, 2H), 7.76 (d, J=7.5, 2H).
References:
DIETRICH, Evelyne;REDDY, Ranga;TANAKA, Kelly;KANG, Ting;LAFONTAINE, Yanick;RAFAI FAR, Adel;TARGANTA THERAPEUTICS CORP. WO2010/19511, 2010, A2 Location in patent:Page/Page column 125-126
28920-43-6
549 suppliers
$5.00/5g

16874-12-7
251 suppliers
$14.00/1G

133852-23-0
62 suppliers
inquiry