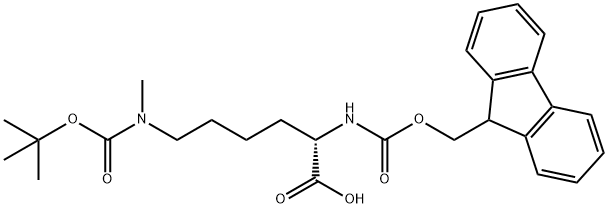
FMOC-LYS(BOC)(ME)-OH synthesis
- Product Name:FMOC-LYS(BOC)(ME)-OH
- CAS Number:951695-85-5
- Molecular formula:C27H34N2O6
- Molecular Weight:482.57

24424-99-5

951695-86-6

951695-85-5
The general procedure for the synthesis of N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N6-(tert-butoxycarbonyl)-N6-methyl-L-lysine from di-tert-butyl dicarbonate and (S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-6-(methylamino)hexanoic acid was performed as follows: the light yellow solid crude product was dissolved in water (500 mL) and THF (500 mL) in a 1:1 solvent mixture, sodium bicarbonate (63 g, 0.75 mol) was added, and the solution was stirred until clarified. Subsequently, Boc2O (142 g, 0.65 mol) was dissolved in tetrahydrofuran (200 mL), added to the reaction system and reacted for 4.0 h at room temperature. Complete reaction of the feedstock was confirmed by HPLC monitoring. After completion of the reaction, the reaction solution was neutralized with dilute hydrochloric acid to pH 5-6. The organic solvent was removed by concentration under reduced pressure and the aqueous phase was extracted with ethyl acetate. The organic phase was washed with saturated brine and concentrated to give a white solid product (235 g). This white solid was further purified by recrystallization from ethyl acetate and n-hexane, resulting in 224 g of white solid powder in 93% yield and 99.8% purity.

24424-99-5
867 suppliers
$13.50/25G

1449300-08-6
0 suppliers
inquiry

951695-85-5
146 suppliers
$52.00/100mg
Yield:951695-85-5 224 g
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 20; for 4 h;Reagent/catalyst;
Steps:
1-5
Use the above pale yellow solid crude product 1: 1 of water (500 mL) and THF (500 mL) dissolved, Add sodium bicarbonate (63 g, 0.75 mol), Stir until clarified, (Boc) 2O (142 g, 0.65 mol) in tetrahydrofuran (200 mL), Room temperature reaction 4.0h, HPLC detection showed complete reaction of the starting material. The reaction solution is neutralized with alkene hydrochloride to a pH of 5-6. The organic solvent was removed by concentration under reduced pressure. The aqueous phase was extracted with ethyl acetate. Washed with saturated saline, The organic phase was concentrated to give a white solid product (235 g). The white solid was recrystallized from ethyl acetate and n-hexane. Obtained 224 g of a white solid powder. The yield was 93% and the purity was 99.8%.
References:
CN108383757,2018,A Location in patent:Paragraph 0019; 0028; 0029; 0033; 0034; 0038; 0039; 0043
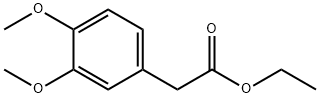
18066-68-7
85 suppliers
$7.00/1g

951695-85-5
146 suppliers
$52.00/100mg
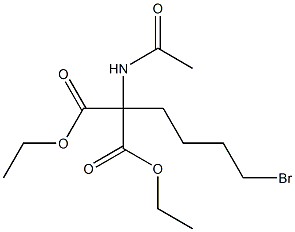
5183-27-7
0 suppliers
inquiry

951695-85-5
146 suppliers
$52.00/100mg
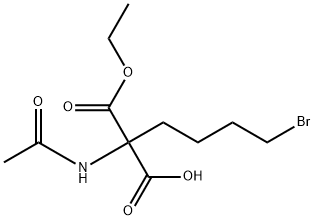
757233-08-2
0 suppliers
inquiry

951695-85-5
146 suppliers
$52.00/100mg
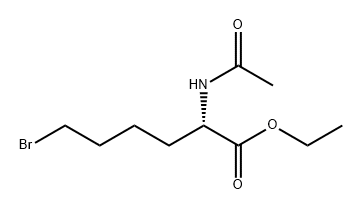
757233-09-3
0 suppliers
inquiry

951695-85-5
146 suppliers
$52.00/100mg