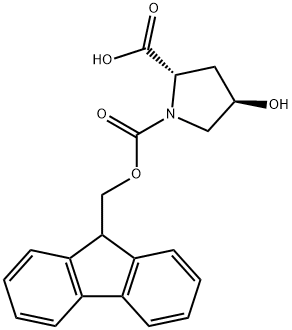
Fmoc-L-hydroxyproline synthesis
- Product Name:Fmoc-L-hydroxyproline
- CAS Number:88050-17-3
- Molecular formula:C20H19NO5
- Molecular Weight:353.37
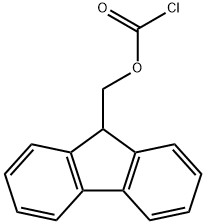
28920-43-6
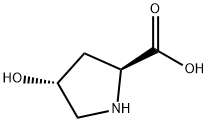
51-35-4

88050-17-3
The general procedure for the synthesis of Fmoc-L-hydroxyproline from methyl 9-fluorenyl chloroformate and L-hydroxyproline is as follows: Example 2: Synthesis of (2S,4R)-1-[(9H-fluoren-9-ylmethoxy)carbonyl]-4-hydroxypyrrolidine-2-carboxylic acid 1. Sodium bicarbonate (80 g) and water (400 ml) were added to a solution of tetrahydrofuran (THF, 200 ml) containing (2S,4R)-4-hydroxy-L-proline (100 g) at 25-30 °C. 2. A solution of 9-fluorenylmethoxycarbonyl (Fmoc) chloride (226 g) dissolved in 200 ml of THF was added slowly. 3. The reaction mixture was stirred continuously at 25-30°C for 10-12 hours until the reaction was complete. 4. Upon completion of the reaction, the reaction was terminated by the addition of an appropriate amount of water. 5. The reaction mixture was washed with diisopropyl ether (DIPE) and subsequently acidified with 1N hydrochloric acid. 6. The acidified mixture was continued to be stirred for 2-3 hours to promote precipitation of the product. 7. The solid product was collected by filtration to give 150 g of Fmoc-L-hydroxyproline as a white solid. Product Characterization: 1H NMR (300 MHz, DMSO-d6): δ 1.89-2.24 (m, 2H), 3.34-3.43 (m, 2H), 3.43-3.54 (m, 0.5H), 4.12-4.21 (m, 3H), 4.25 (s, 2H), 4.28-4.42 (m, 0.5H), 5.16 (brs, 1H) , 7.29-7.34 (m, 2H), 7.38-7.65 (m, 2H), 7.63-7.65 (m, 2H). Melting point: 188-190°C. Mass spectrum (M + H): 354.33.
Yield:88050-17-3 150 g
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 25 - 30;
Steps:
2 Synthesis of (2S, 4R)-1-[(9H-fluoren-9-ylmethoxy) carbonyl]-4-hydroxypyrrolidine-2-carboxylic acid
Example 2
Synthesis of (2S, 4R)-1-[(9H-fluoren-9-ylmethoxy) carbonyl]-4-hydroxypyrrolidine-2-carboxylic acid
To a solution of (2S,4R)-4-hydroxy-L-proline (100 g) in tetrahydrofuran (200 ml) was added sodium bicarbonate (80 g), water (400 ml) and 9-fluorenylmethyloxycarbonyl (Fmoc) chloride (226 g) solution (in 200 ml THF) at 25-30° C.
The reaction mixture was stirred at about 25-30° C. for about 10-12 h.
After completion of reaction, water was added.
Then the aq.
reaction mass was washed with diisopropyl ether (DIPE) and acidified with 1N hydrochloric acid.
The reaction mixture was stirred for about 2-3 hours.
The solid was collected by filtration to give the 150 g of title compound as white solid.
1H NMR (300 MHz, DMSO-d6): δ 1.89-2.24 (m, 2H), 3.34-3.43 (m, 2H), 3:43-3.54 (m, 0.5H), 4.12-4.21 (m, 3H), 4.25 (s, 2H), 4.28-4.42 (m, 0.5H), 5.16 (brs, 1H), 7.29-7.34 (m, 2H), 7.38-7.65 (m, 21-1), 7.63-7.65 (m, 2H), 7.87-7.89 (m, 2H)
Melting point:-188-190° C.
Mass-(M+H):-354.33
References:
Glenmark Pharmaceuticals Limited;Kadam, Suresh Mahadev;Kansagra, Bipin Parsottam;Kale, Ramchandran Vishnue;Patil, Jayant Prakashrao;Yemireddy, Venkataramana Reddy;Bhadane, Shailendra Nilkanth;Chaudhar, Uddhav Popat;Patil, Ulhas Digambar;Bhirud, Shekhar Bhaskar US9518048, 2016, B2 Location in patent:Page/Page column 24
82911-69-1
648 suppliers
$7.00/25g

88050-17-3
244 suppliers
$5.00/1g