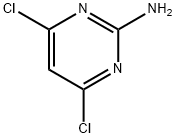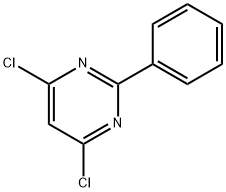
Fenclorim synthesis
- Product Name:Fenclorim
- CAS Number:3740-92-9
- Molecular formula:C10H6Cl2N2
- Molecular Weight:225.07

13566-71-7

3740-92-9
The general procedure for the synthesis of 4,6-dichloro-2-phenyl-pyrimidine from 2-phenylpyrimidine-4,6-diol was as follows: 300 ml of phosphoryl chloride was added to 40 g of 2-phenylpyrimidine-4,6-diol. The reaction mixture was heated to reflux at 100 °C for 48 hours. Upon completion of the reaction, the excess phosphoryl chloride was removed by distillation under reduced pressure (20 mbar). Subsequently, 200 ml of chloroform and 100 ml of ice water were added to the reaction mixture and stirred thoroughly for 30 minutes. The pH of the mixture was adjusted to 5-6 with aqueous sodium carbonate. the organic layer was separated and the aqueous phase was extracted three times with 200 mL of chloroform. All chloroform phases were combined, dried with magnesium sulfate, filtered and the chloroform was removed by evaporation under reduced pressure. The resulting crude product can be purified by distillation under reduced pressure or by column chromatography using 60 mesh silica gel (with chloroform as eluent).

13566-71-7
53 suppliers
$9.00/250mg

3740-92-9
242 suppliers
$8.00/10g
Yield:3740-92-9 95%
Reaction Conditions:
in trichlorophosphate
Steps:
1 Synthesis of Chloroacetamide Intermediate 6.
4,6-Dichloro-2-phenylpyrimidine (3): A slurry of diol 2 (39.08 g; 0.208 mol) in phosphorus oxychloride (124 mL; 1.33 mol) was heated from 75-120° C. over 45 min. After 20 h at 120° C. the volatiles were removed in vacuo. The resulting tan solid was added to crushed ice (600 mL) and this suspension was stirred for 2 h at rt, collected by filtration, washed with water and dried to a constant weight under high vacuum giving a tan solid (44.34 g; 95%). 1H NMR (200 MHZ, CDCl3) δ 8.44 (m, 2H), 7.51 (m, 3H), 7.27 (s, 1H).
References:
Castelhano, Arlindo;McKibben, Bryan;Steinig, Arno;Collington, Eric US2003/162764, 2003, A1

13566-71-7
53 suppliers
$9.00/250mg

3740-92-9
242 suppliers
$8.00/10g
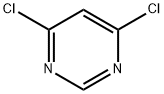
1193-21-1
575 suppliers
$13.00/5g
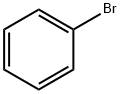
108-86-1
525 suppliers
$10.00/5g

3740-92-9
242 suppliers
$8.00/10g
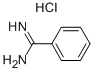
1670-14-0
435 suppliers
$6.00/10g

3740-92-9
242 suppliers
$8.00/10g