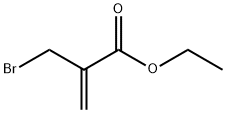
ETHYL 2-(BROMOMETHYL)ACRYLATE synthesis
- Product Name:ETHYL 2-(BROMOMETHYL)ACRYLATE
- CAS Number:17435-72-2
- Molecular formula:C6H9BrO2
- Molecular Weight:193.04
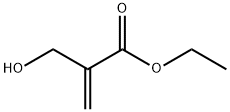
10029-04-6

17435-72-2
GENERAL STEPS: Phosphorus tribromide (0.51 mL, 5.4 mmol) was added slowly and dropwise to a stirred solution of ethyl 2-hydroxymethacrylate (2 g, 15.4 mmol) in ether (15 mL) at 0 °C. The reaction mixture was stirred at 0 °C for 3 h and subsequently warmed to room temperature. The reaction was quenched by the addition of water (5 mL) and the product was extracted with hexane (3 x 10 mL). The organic layers were combined, washed with brine (2 × 10 mL), dried over anhydrous MgSO4, filtered and concentrated in vacuum to afford ethyl 2-bromomethacrylate (2.4 g, 12.3 mmol, 82% yield) as a colorless liquid, which could be used in the next reaction without further purification. The product was characterized as follows: colorless oil; 1H NMR (400 MHz, CDCl3) δ 1.30 (t, J = 7.2 Hz, 3H, H6), 4.16 (s, 2H, H4), 4.24 (q, J = 7.0 Hz, 2H, H5), 5.92 (s, 1H, H1'), 6.30 (s, 1H, H1); 13C NMR ( 101 MHz, CDCl3) δ 14.1 (CH3, C6), 29.3 (CH2, C4), 61.2 (CH2, C5), 128.8 (CH2, C1), 137.6 (C, C2), 164.8 (C, C3); IR (νmax, cm-1) 1718 (C=O ester), 1628 (C=C alkene), 1182 (C-O ester), 523 (C-Br); HRMS-EI: m/z [M+H]+ calcd for C6H10BrO2: 192.9864, found: 192.6870.
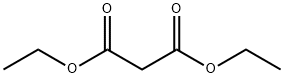
105-53-3
786 suppliers
$5.00/25g

17435-72-2
200 suppliers
$9.00/1g
Yield:-
Reaction Conditions:
in acetonitrile
Steps:
P.1 [Formula (IA), X = COOCH2CH3; Y = R3 = R4 = H; R1 = R2 = CH2CH3].
Preparative Example 1 Ethyl 2,4-bis(ethoxycarbonyl)-4-pentenoate (Ia) [Formula (IA), X = COOCH2CH3; Y = R3 = R4 = H; R1 = R2 = CH2CH3]. [Typical procedure]. To a suspension of sodium hydride (80% dispersion in oil, 0.36g, 12mmol) in acetonitrile (10mL), was added diethyl malonate (1.60g, 10mmol). The resulting suspension was allowed to stir at room temperature for 15 minutes. A solution of ethyl α-(bromomethyl)acrylate [obtained from a modified procedure of S. E. Drewes, G. Loizou and G. H. P. Roos, Synthetic Communications, 1987, 17(3) , 291-298] (1.93g, 10mmol) in acetonitrile (5mL) was then added slowly to the above suspension. Stirring was maintained for 2 hours and then the reaction mixture was poured into water, and extracted (3x) with diethyl ether.
References:
COMMONWEALTH SCIENTIFIC AND INDUSTRIAL RESEARCH ORGANISATION;E.I. du Pont de Nemours and Company (a Delaware corporation) EP729449, 2000, B1

10029-04-6
116 suppliers
$15.00/250mg

17435-72-2
200 suppliers
$9.00/1g

64-17-5
758 suppliers
$10.00/50g

72707-66-5
101 suppliers
$72.00/250mg

17435-72-2
200 suppliers
$9.00/1g
140-88-5
551 suppliers
$12.00/100ml

17435-72-2
200 suppliers
$9.00/1g
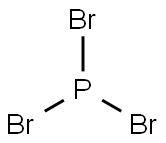
7789-60-8
397 suppliers
$10.40/25G

17435-72-2
200 suppliers
$9.00/1g